Psoriasis
Latest News

Latest Videos

CME Content
More News
Matthew Zirwas, MD, discusses the complexities of safety analysis in psoriasis clinical trials, advocating for exposure-adjusted incidence rates to enhance risk communication.

Johnson & Johnson submitted an NDA for icotrokinra, a promising new treatment for moderate to severe plaque psoriasis in adults and adolescents.
A retrospective case series found that apremilast induced complete remission in 6 men with treatment-resistant genital psoriasis.

At a recent Dermatology Times Case-Based Roundtable event, David Cotter, MD, PhD, reviewed treatment considerations for older patients with moderate to severe PsO who are candidates for biologic therapy.

The pharmaceutical companies announced an exclusive global license and transfer agreement for the advancement and commercialization of Spevigo.
Spesolimab effectively targets the IL-36 receptor, reducing inflammation and preventing flares in generalized pustular psoriasis, offering new hope for patients.

Although bimekizumab carries a precaution for suicidal ideation and behavior, current data suggest it may improve mental health outcomes for patients with psoriasis.

VYNE Therapeutics updates on VYN202, revealing promising early results in psoriasis treatment despite a clinical hold due to safety concerns.
An expert discusses how managing psoriasis requires personalizing treatment based on patient adherence challenges, access issues, comorbidities such as depression, and practical considerations such as injection comfort and frequency.

A poster from SDPA 2025 showed brodalumab maintains a consistent safety profile with no completed suicides and low infection rates.
Subcutaneous spesolimab maintained durable GPP control for 12 months after rapid clearance from IV spesolimab in a high-risk elderly patient.
Remission is now defined as 0% BSA or IGA 0 for ≥6 months on treatment, per a Delphi consensus led by the National Psoriasis Foundation.

Explore the latest insights on IL-17 inhibitors for psoriasis, highlighting their mechanisms, real-world benefits, and future treatment strategies.

New research reveals that early discontinuation of adjuvant immunotherapy for melanoma does not lead to worse outcomes.

Aaron Farberg, MD, led a panel exploring the challenges of diagnosing and treating generalized pustular psoriasis and the promise of targeted therapies.
Zasocitinib emerges as a promising oral therapy for psoriasis and psoriatic arthritis, showcasing superior selectivity and safety compared to traditional JAK inhibitors.

The National Psoriasis Foundation Seal of Recognition program has expanded to prescription drugs, making roflumilast the first to achieve the indication.

Ben Lockshin, MD, FAAD, covered ways in which GLP-1 RAs can be utilized when treating psoriatic disease.

Put your dermatology expertise to the test with Dermatology Times’ interactive quiz series!

Celltrion's Yuflyma gains full FDA interchangeability, enhancing patient access and affordability for various inflammatory conditions.

FDA approves Arcutis' roflumilast foam, a groundbreaking treatment for plaque psoriasis, offering a steroid-free, effective solution for patients aged 12 and older.

A recent analysis reveals safety profiles of apremilast and deucravacitinib for psoriasis, highlighting unique adverse events and long-term monitoring needs.
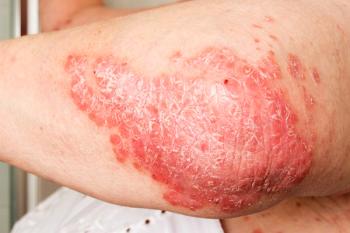
Ascletis Pharma's investigational new drug application for ASC50 gains FDA clearance for a phase 1 trial for mild to moderate psoriasis.

Accropeutics reveals promising Phase 2 trial results for AC-201, a selective TYK2/JAK1 inhibitor, showing significant efficacy in treating plaque psoriasis.

Psoriasis significantly impacts sleep health, with diverse populations facing increased risks of sleep disorders, highlighting urgent clinical needs for tailored interventions.