Psoriasis
Latest News

Latest Videos

CME Content
More News
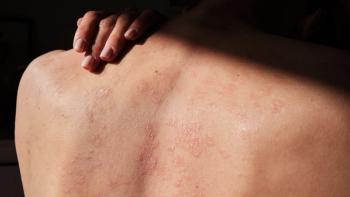
At Fall Clinical, new findings from the ENCOMPASS study revealed significant unmet needs in psoriasis care, highlighting a strong preference for effective oral therapies among patients and providers.
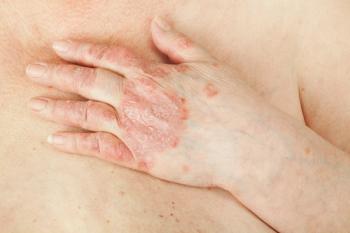
Bristol Myers Squibb reveals promising data on deucravacitinib's efficacy for psoriatic arthritis and systemic lupus erythematosus, highlighting its potential as a new treatment option.

Benjamin Lockshin, MD, noted long half-life biologics may allow patients to maintain remission with minimal injections.

At Fall Clinical 2025, Ron Vender, MD, discussed the evolving psoriasis treatment landscape and the persistent challenge of managing palmoplantar pustulosis.

At Fall Clinical 2025, Marc Serota, MD, discussed how GLP-1 therapies, new CSU treatments, and hybrid care models are reshaping dermatologic practice heading into 2026.

New data presented at Fall Clinical 2025 reveal that patients in socioeconomically disadvantaged areas are significantly less likely to initiate biologic or small molecule treatments.

New data from Johnson & Johnson's ICONIC-TOTAL study reveals icotrokinra's promising long-term efficacy and safety for treating challenging plaque psoriasis.

Data from Fall Clinical 2025 detailed the results from the phase 2 STRIDE and ongoing open-label extension studies demonstrating the efficacy and safety of envudeucitinib, a selective TYK2 inhibitor.

A new IPC-endorsed framework simplifies psoriasis severity into “topical” versus “systemic” categories to guide treatment more effectively, according to James Song, MD.
James Del Rosso, DO; April Armstrong, MD, MPH; Mark Nestor, MD, PhD; Mark Lebwohl, MD; Linda Stein Gold, MD; and Darrell Rigel, MD, MS, presented some of the biggest updates in dermatology to close out the year.

Explore real-world strategies with Leigh Ann Pansch, MSN, FNP-BC, DCNP, for managing psoriasis, emphasizing patient-centered care, and implementing individualized biologic treatment approaches.

Vimal Prajapati, MD, FRCPC, DABD, highlights the durable efficacy of guselkumab, reassuring safety, and quality-of-life improvements in pediatric patients with moderate to severe plaque psoriasis.

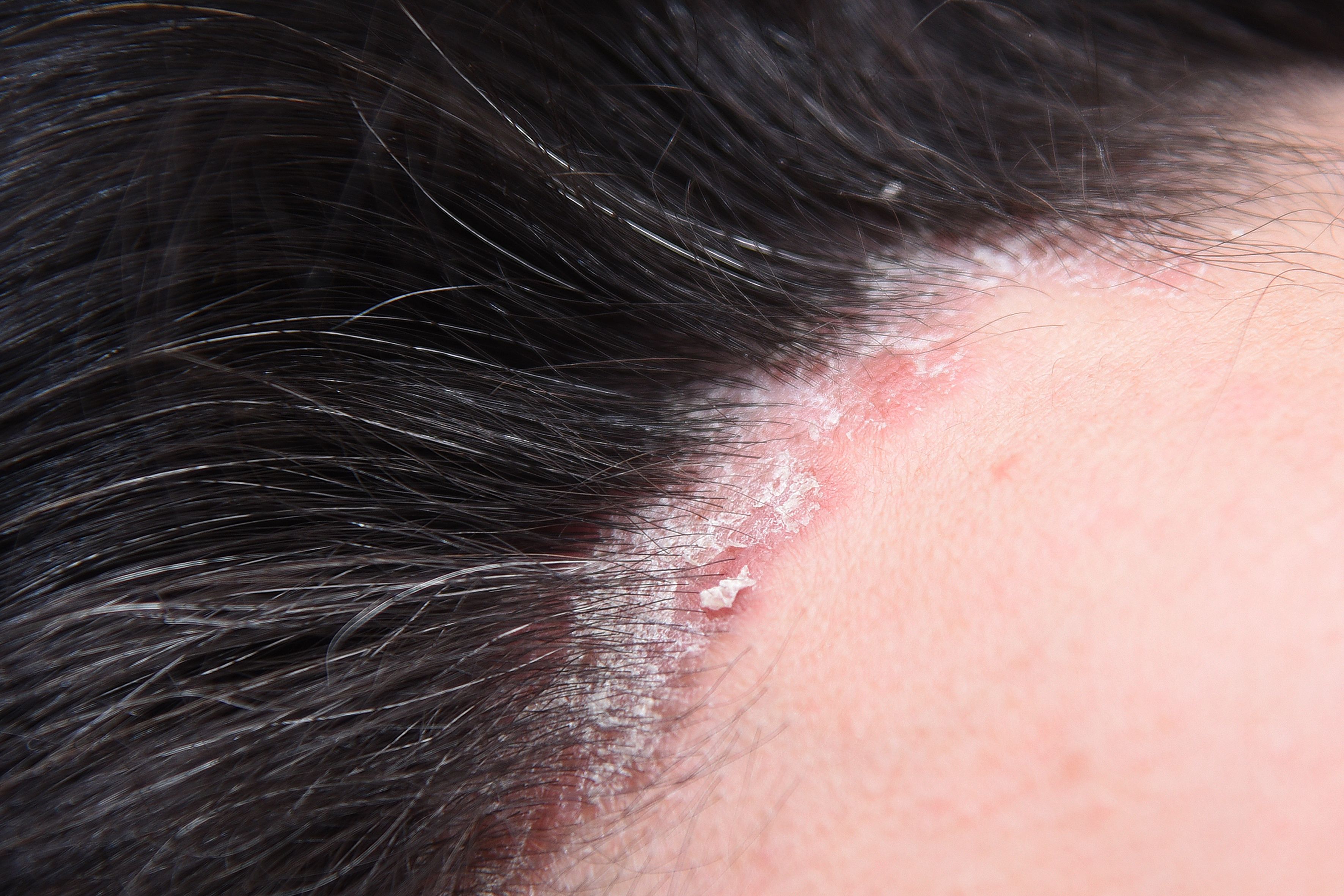
Andrew Mastro, MS, PA-C, moderated a recent Dermatology Times Case-Based Roundtable event to present 3 complex cases of moderate to severe psoriasis treated with biologics.

Vimal Prajapati, MD, FRCPC, DABD, highlights guselkumab’s ability to achieve high levels of skin clearance in pediatric patients with moderate to severe plaque psoriasis.

Mark Kaufmann, MD, discusses how patient realities shape psoriasis treatment choices.

Researchers found new patients incurred 40% higher consultation costs due to longer clinician interaction times.

A study reveals ixekizumab's effectiveness in treating mild to moderate plaque psoriasis, showing superior PASI responses and improved long-term outcomes.

Psoriasis complicates wound healing due to systemic inflammation, affecting recovery and requiring tailored clinical approaches for optimal patient outcomes.

Explore real-world psoriasis management insights from Anthony Nuara, MD, PhD, focusing on patient-centered approaches and innovative treatments like tildrakizumab.

Discover the latest IPC consensus on psoriasis severity reclassification, enhancing treatment strategies for better patient outcomes and quality of life.
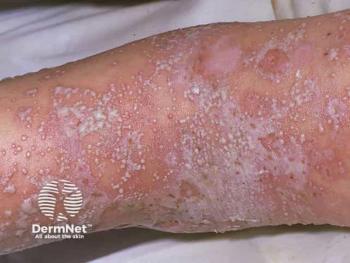
Spesolimab joins LEO Pharma’s dermatology portfolio to further expand spesolimab’s reach for patients worldwide with generalized pustular psoriasis.

WHO includes adalimumab and ustekinumab for psoriasis, plus sunscreen for albinism, enhancing global skin health and access to essential treatments.

ORKA-001 builds on the proven success of existing IL-23 inhibitors used in psoriasis treatment.

Both treatments were well tolerated, with only mild, self-limiting adverse effects reported.

Phase 3 PROTOSTAR data support new indications, expanding treatment options for children impacted by chronic immune-mediated diseases.