
The submission follows the announcement of recent long-term data of the drug in patients ages 6 years and older.

The submission follows the announcement of recent long-term data of the drug in patients ages 6 years and older.

Both companies are hoping to make a positive impact in the neoadjuvant setting of cutaneous squamous cell carcinoma.
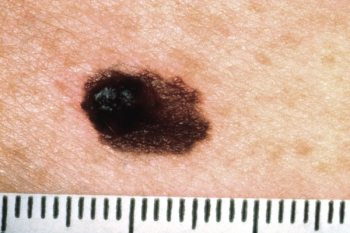
V940-001 is the first phase 3 study of a planned comprehensive clinical development program initiated after the positive primary analysis of the phase 2b KEYNOTE-942/mRNA-4157-P201 trial.

Both therapies for alopecia areata and completely resected stage IIB/C melanoma could be approved by the EC for patients aged 12 years or older.

Verrica’s VP-102 is the first FDA-approved treatment for molluscum lesions.

The goal of a January 5, 2024, PDUFA date for berdazimer gel, 10.3%, continues with pre-approval inspection already complete.
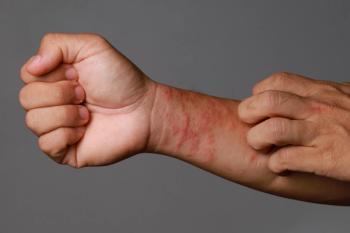
AB-101a is a new topical treatment being investigated for mild, moderate, and severe atopic dermatitis and its immune system component.

Coverage for eligible patients began July 12, 2023.

The TRuE-AD3 study met its primary endpoint of IGA-TS improvement from baseline at week 8.
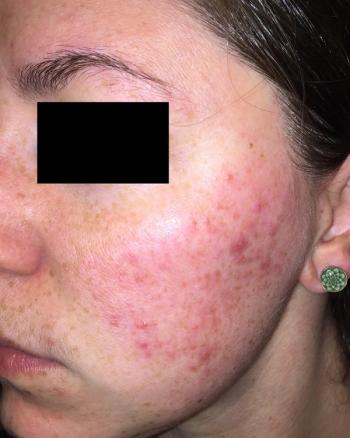
Based on the positive topline phase 3 results, Journey Medical plans to submit a New Drug Application to the FDA in the second half of 2023.

New pharmacokinetic phase 3 data will be presented at the 2023 Society for Pediatric Dermatology meeting next week.

It was found that cancer treatment, pediatric population, and coverage restriction of reference products were revealed as some of the strongest factors associated with biosimilar coverage decisions by US commercial health plans relative to reference products.

Boehringer Ingelheim’s Cyltezo is the first and only FDA-approved interchangeable biosimilar to adalimumab.

Sanofi’s investigational anti-OX40-ligand monoclonal antibody showed statistically significant improvement in signs and symptoms of AD in adults.

The Act4Biosimilars Action Plan aims to highlight key challenges preventing patient access to biosimilars and outline steps to help overcome them.

The current dermatology pipeline includes a wide range of medication classes, from Janus kinase (JAK) inhibitors to tumor necrosis factor inhibitors, to phototherapeutics

CEO Daniela Marino, PhD, emphasizes that the Switzlerand based company has received Orphan Drug Designation from the Swissmedic, EMA, and FDA, for its personalized human skin graft.
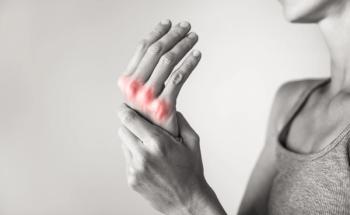
Apremilast is being examined for the treatment of psoriatic arthritis.

Yuflyma will be available to US patients in July 2023.

The adalimumab biosimilar will be commercially available in the US starting July 1, 2023.

Beremagene geperpavec is the first FDA-approved treatment for DEB, a rare and burdensome disease.

ASLAN Pharmaceuticals will present 2 late-breaking abstracts and 2 additional abstracts.

Ruxolitinib is the first and only approved treatment for repigmentation in the European Union.

Approval has been pushed back to June 28, 2023.

Ritlecitinib was found to be effective and well-tolerated in patients aged 12 years and older.