
Patrick Burnett, MD, PhD, provides insights into the significance of Arcutis’ roflumilast cream 0.15% formulation and its impact on pediatric patients.

Patrick Burnett, MD, PhD, provides insights into the significance of Arcutis’ roflumilast cream 0.15% formulation and its impact on pediatric patients.

The planned PDUFA data was July 7, 2024.

Iain Stuart, PhD, discusses VYN202's status and potential in psoriasis and immuno-inflammatory diseases.

Pyzchiva will be commercialized by Sandoz in the United States.

Ensemble No.2, or ENS-002, is an investigational live biotherapeutic product first detected by Concerto Biosciences using its kChip technology.

Authorization for Eli Lilly's Ebglyss is based on positive results from the ADvocate 1, ADvocate 2, and ADhere studies.

Botanix Pharmaceuticals announced the approval of its sofpironium topical gel, 12.45% for adults and children ages 9 and older.

Stefan Weiss, MD, MBA, explains next steps for the oral treatment after the completion of phase 1b enrollment.

LEO Pharma's Adbry, a single-dose autoinjector, is expected to become available to patients in the coming months.

Roflumilast cream 0.15% is being evaluated for patients with mild to moderate atopic dermatitis down to 6 years.

Researchers found dupilumab improved itch and skin lesions regardless of atopic disease status in patients with PN.

The primary efficacy endpoint is the proportion of subjects achieving at least a 50% improvement in the Facial Vitiligo Area Scoring Index.

Phase 3 trials have been initiated in patients with high-risk melanoma.
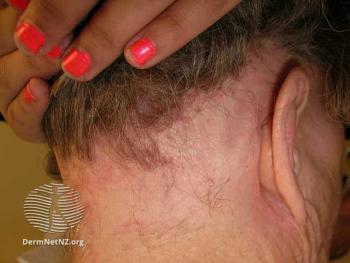
The phase 2 study achieved the primary end point of a >30% Severity of Alopecia Tool score improvement.

The month of May has been full of pipeline news, including updates on VYN201 for nonsegmental vitiligo, imsidolimab for GPP, sonelokimab for HS, and more.

The Lancet data is the primary publication of bimekizumab results from BE HEARD I and BE HEARD II.

The new action date is December 29, 2024.

Scassellati Sforzolini discusses the significance of Galderma’s nemolizumab receiving 4 additional filing acceptances for prurigo nodularis and atopic dermatitis.
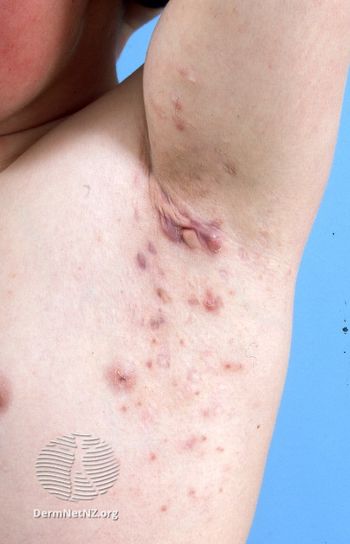
VELA is the first phase 3 hidradenitis suppurativa trial to evaluate HiSCR75 as the primary end point.

Bristol Myers Squibb announced 70% of patients maintained significant improvement after 4 years with no new safety concerns.

Acinonyx Bio's topical cream, ACX, targets Propionibacterium acnes.
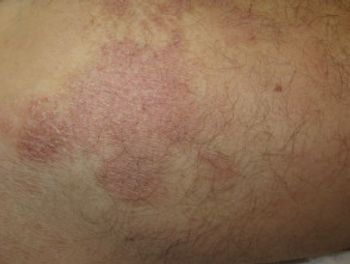
The results are both confirmatory and extend the response results from a prior phase 3 trial known as FLASH.

The analysis revealed a higher rate of discontinuations due to immune-mediated adverse experiences.

Patients who responded to imsidolimab in GEMINI-1 and who moved into GEMINI-2 maintained clear or almost clear skin.
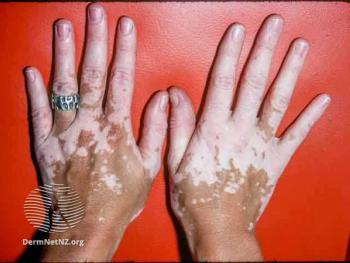
VYNE expects to enroll the first patient with vitiligo in the phase 2b trial for VYN201 in the second quarter of 2024.