
The company was asked by the FDA to submit additional confirmatory evidence of effectiveness in the treatment of epidermolysis bullosa.

The company was asked by the FDA to submit additional confirmatory evidence of effectiveness in the treatment of epidermolysis bullosa.

A recent mouse model study on the efficacy of using imatinib as a treatment for Sturge Weber Syndrome has been published. In this video interview we talk with one of the investigators, Jack L. Arbiser, MD, PhD, on what the results means for future clinical trials.

Timber Pharmaceuticals announced a positive end-of-phase 2 meeting with the FDA about TMB-001 in moderate to severe congenital ichthyosis. Timber also revealed when it plans to start a phase 3 trial.

Allergan Aesthetics, an AbbVie company, announced the FDA approval of Juvéderm Volbella XC for undereye hollows.

AbbVie announced the FDA approval to their IL-23 inhibitor risankizumab-rzaa (Skyrizi) for treatment of active psoriatic arthritis (PsA) in adult patients based on the results of the KEEPsAKE-1 and KEEPsAKE-2 trials.
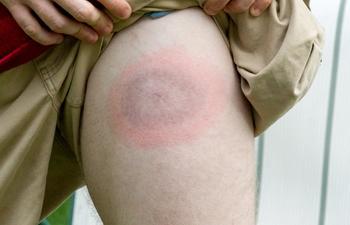
What new treatments are coming to infectious disease? How can they be prevented and what misconceptions are affecting treatment? Learn about this and more in this video with Ted Rosen, MD.

In this video, Peter Lio, MD, FAAD, expands on his presentation at the 19th annual Winter Clinical Dermatology Conference by diving into molluscum contagiousum treatment and the potential of cantharidin 0.7% (VP-102; Verrica).

Vial launches Vial Dermatology contract research organization (CRO) and highlights how it could improve the speed and quality of clinical trials.

The new year promises to be rich with dermatology game changers following a sluggish 2021 for breakthroughs and FDA approvals.

Skin sensitivity will be a factor in vehicles for new treatments.

Click here to answer this week's poll.

The past year gave dermatologists ample cause for both celebration and frustration as they looked forward to new drugs, devices, and technology to optimize patient care. Here, Dermatology Times® editorial advisory board members weigh in on the good, bad, and still-TBD changes that shaped the specialty during 2021.

Verrica Pharmaceuticals announced the FDA acceptance of the New Drug Application (NDA) resubmission for VP-102 (Verrica Pharmaceuticals) for the treatment of molluscum contagiosum.

The FDA grants Priority Review for spesolimab (BI655130; Boehringer Ingelheim) as a treatment for generalized pustular psoriasis.

The FDA approves upadacitinib (Rinvoq; AbbVie) for the treatment of adult patients with active psoriatic arthritis.

Ruxolitinib cream (Opzelura; Incyte) receives priority review of its supplemental New Drug Application as a treatment for patients with vitiligo.

Arcutis Biotherapeutics has announced data suggesting that the vehicle in the investigational roflumilast cream had similar moisturizing properties as a commercially marketed, dermatologist-recommended, ceramide-containing moisturizer in adults with mild eczema.

In this exclusive video interview, James Q Del Rosso, DO, gets into the details of JAK inhibitors, explaining how they work and cutting through the headlines around the treatments.

We sit down in a video interview with David Pariser, MD, FACP, FAAD, the secretary and founding member of the International Hyperhidrosis Society to discuss hyperhidrosis identifiers and treatments in honor of Hyperhidrosis Awareness Month.
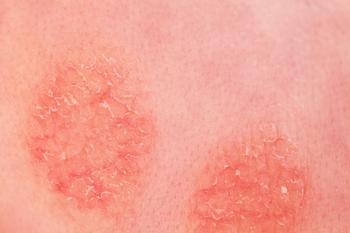
FDA approval of ruxolitinib (Opzelura, Incyte) adds the first new topical anti-inflammatory to the atopic dermatitis (AD) armamentarium in 50 years, but boxed warnings raise questions.

Potential game-changers in the pipeline and newly FDA-approved treatments were highlighted in an overview of new drugs to watch at Maui Derm NP+PA Fall 2021 meeting.
Galderma and Sofregen Medical announced they have entered into an agreement to co-develop a novel line of silk-based biostimulators that will enable immediate volume restoration and provide structure for new tissue generation.

New therapies raise patient expectations for fast efficacy, lower costs, and fewer adverse effects.

This episode highlights findings from an investigative study exploring the molecular targeting of biologics for psoriasis, the IL-17 drug class, how bimekizumab is different, plus more.
Possible melanoma treatments have had multiple newsworthy events since July 2021.