
The innovative technology is unlocking new possibilities in dermatology by improving the solubility and permeability of drugs for topical use.

The innovative technology is unlocking new possibilities in dermatology by improving the solubility and permeability of drugs for topical use.

Dermatology Times is reviewing some of the most anticipated PDUFA dates of the upcoming year.
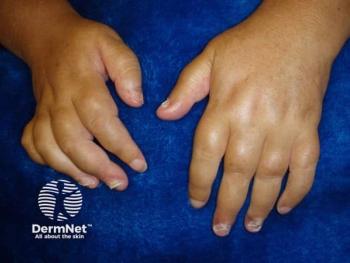
Deucravacitinib achieved ACR20 response in patients with PsA at week 16, with a safety profile consistent with previous studies.
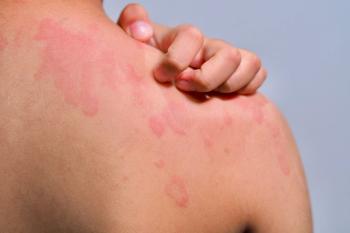
The company stated INF904 disrupts inflammatory pathways with over 90% effectiveness, based on Phase 1 data.

If granted, guselkumab will be approved to treat children ages 6 and younger with severe plaque psoriasis and children ages 5 and younger with juvenile psoriatic arthritis.

According to the company, comparative tests revealed PN-881 surpasses or matches the efficacy of current injectable IL-17 therapies.

The drug is the first FDA-approved treatment for moderate to severe HS that selectively inhibits both IL-17A and IL-17F.

Delgocitinib is indicated for adults with CHE who either do not respond to or cannot use topical corticosteroids.

Abeona's pz-cel has been granted a April 29, 2025, PDUFA date.

The target action review date has been pushed to March 12, 2025.

This represents the second approval of Zoryve outside the US.

Light delves into the science behind the new peer-reviewed data assessing benzene presence and formation in benzoyl peroxide products.

New peer-reviewed research published in the Journal of Investigative Dermatology examined 111 products at room temperature.

Key highlights of the presentations included data on delgocitinib for atopic hand eczema, and tralokinumab for head and neck atopic dermatitis.

Through collaboration with Nimbus Therapeutics and Schrodinger, zasocitinib’s AI-driven design maximizes its fit within the targeted enzyme.

The FDA has set a PDUFA target action date of May 22, 2025.

Bimzelx was also approved for non-radiographic axial spondyloarthritis and ankylosing spondylitis.

Ebglyss is now approved for children and adults aged 12 years and older.

The rates of discontinuation due to inefficacy or adverse events for biosimilars and originators of etanercept and adalimumab were similar, according to an analysis of a prospective registry.
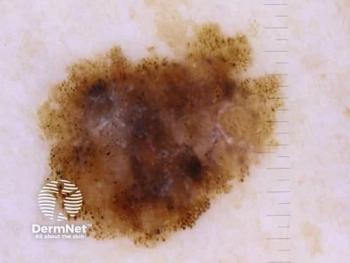
The PD-1/IL-2α bispecific antibody fusion protein is intended for patients with unresectable locally advanced or metastatic melanoma.

The overall reduction of tumor size in all lesions treated in part 2 was approximately 86%.

Galderma’s nemolizumab is the first approved monoclonal antibody specifically inhibiting the signaling of IL-31.

The approval is supported by positive phase 3 data for Lymphir in this indication.

Alumis' Martin Babler shared insights into the program for the TYK2 inhibitor and the company's next steps for its development.

This article represents the first time the news has been shared with the public. Turn's Founder and CEO, Bradley Burnam, shares details.