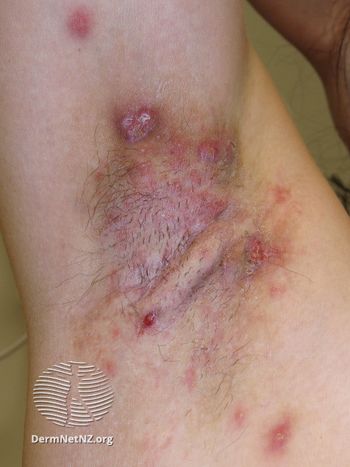
MoonLake’s sonelokimab is being evaluated for hidradenitis suppurativa, psoriasis, and psoriatic arthritis.

MoonLake’s sonelokimab is being evaluated for hidradenitis suppurativa, psoriasis, and psoriatic arthritis.
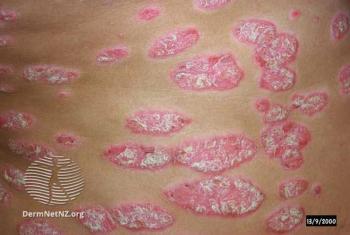
Loretta Fiorillo, MD, FRCPC, reviews key data points from the phase 3 SPROUT study.
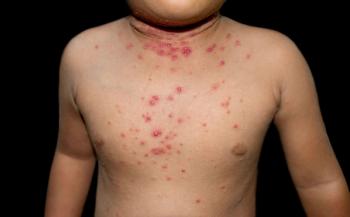
The new launch is set to bring berdazimer topical gel, 10.3% to adults and pediatric patients over the age of 1 with molluscum contagiosum faster.

The merger will focus on advancing Oruka’s portfolio of novel biologics for the treatment of chronic skin diseases.
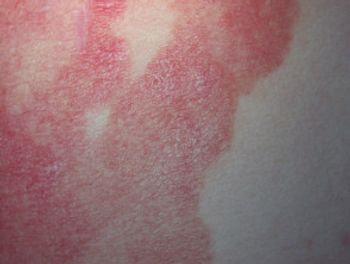
The agreement for a second confirmatory trial comes after a successful phase 3 FLASH study that yielded positive outcomes and efficacy.

The biosimilar landscape experienced significant activity, marked by fresh data from conferences, novel findings regarding adalimumab biosimilars, and more.

Povorcitinib is being evaluated for non-segmental vitiligo, hidradenitis suppurativa, prurigo nodularis, asthma, and chronic spontaneous urticaria.

If approved, bimekizumab will be the first IL-17A and IL-17F inhibitor approved in Europe for hidradenitis suppurativa.

The FDA has assigned a Prescription Drug User Fee Act target action date for August 13.

The anticipated PDUFA date is November 4, 2024.

Shawn Kwatra, MD, shares positive updates on phase 2 oral povorcitinib for prurigo nodularis at AAD 2024.

New data was presented at the American Academy of Dermatology Annual Meeting in San Diego, California.
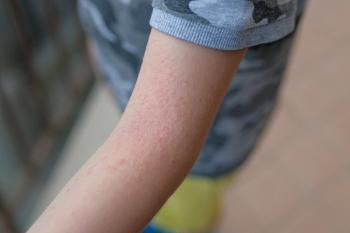
Alphyn’s first-in-class therapeutic is designed to treat the immune system component, and more specifically the bacterial component, of AD.

Miller, Vice President, Immunodermatology Disease Area Leader, Johnson & Johnson, spoke with Dermatology Times about recently published data supporting JNJ-2113's potential in this indication.

Lifileucel is a tumor-derived autologous T cell immunotherapy.

The FDA has also granted nemolizumab Priority Review for prurigo nodularis.

Tapinarof cream demonstrated highly statistically significant improvement in the primary end point of vIGA-AD treatment success over vehicle at week 8.

Data from the FRONTIER 1 clinical trial were recently published in the New England Journal of Medicine.

Marcelo Bigal, MD, PhD, discusses VENT-03's potential as a small molecule therapeutic in dermatology, among other fields.

The VISIBLE clinical program will create a longitudinal collection of more than 20,000 clinical images across all skin tones.

The high-concentration and citrate-free biosimilar was first available in a single 40 mg dose.
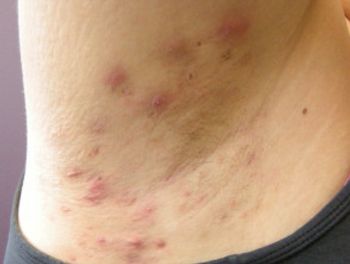
Higher response rates were observed in patients treated with lutikizumab. The drug will now advance to phase 3 clinical trials.

The approval marks a milestone for patients ages 1 year of age and older who face the persistent, highly contagious condition.

The filing is supported by positive phase 3 clinical trial data.
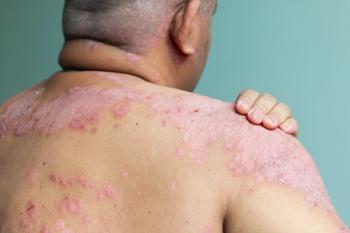
Soligenix shared today positive top-line data stemming from the phase 2a clinical trial.