Drug Watch
Latest News
Latest Videos
CME Content
More News
While approved for vitiligo and atopic dermatitis, ruxolitinib cream may help treat other inflammatory skin diseases.

Exclusive Q1 Report: A comprehensive look at the latest FDA approvals and pipeline developments in dermatology from the first quarter of 2025.

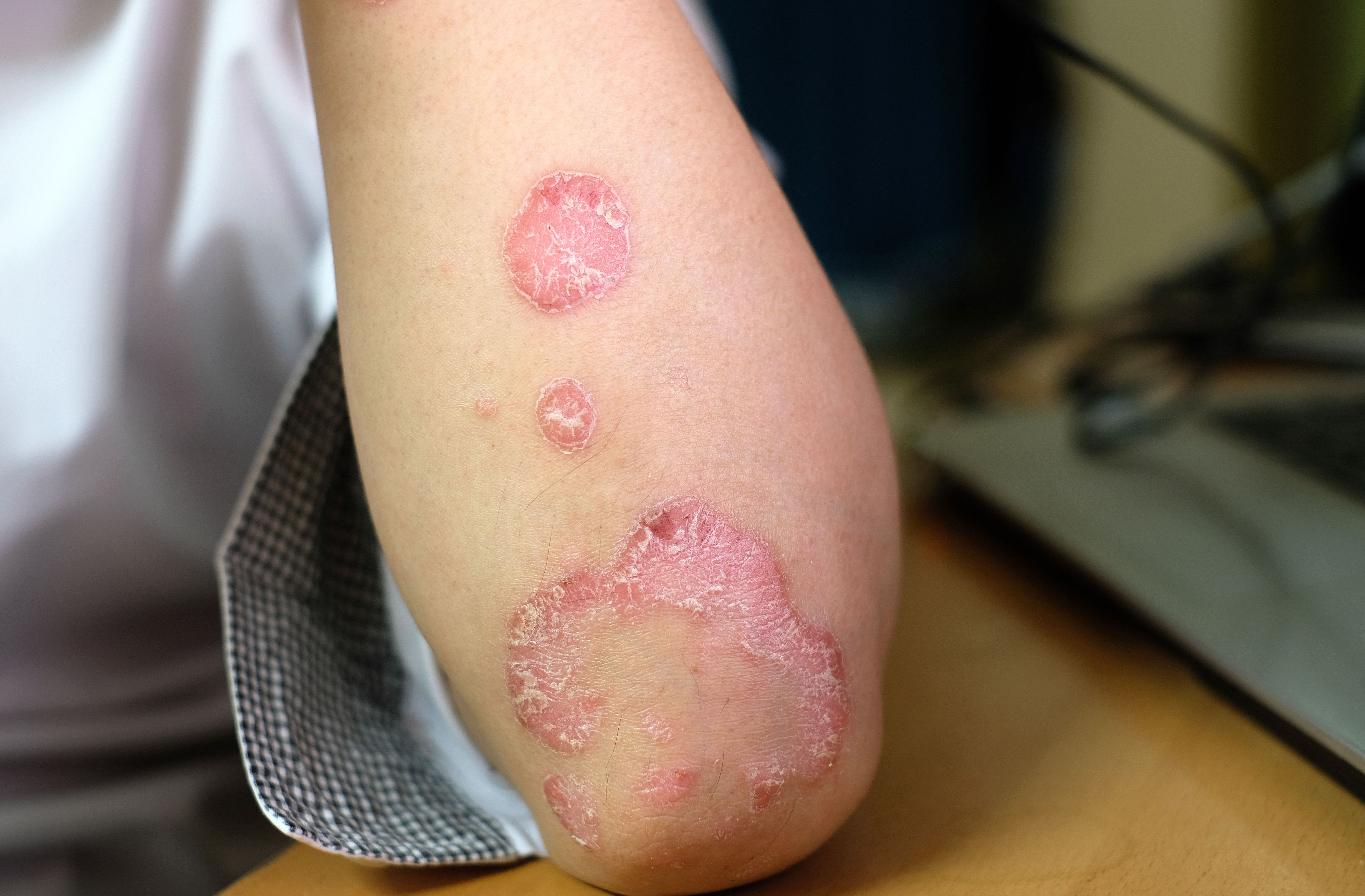
The novel treatments will be for patients with orphan conditions like Netherton Syndrome, Peeling Skin Syndrome, SAM Syndrome, and palmoplantar keratoderma.

A pilot study revealed that mitochondrial complex I-blocking drugs, like metformin and berberine, improved TSW symptoms in most participants.

Christopher Bunick, MD, PhD, discussed how to interpret recent benzene research as clinicians await regulatory guidance for benzoyl peroxide.

Christopher Bunick, MD, PhD, explains what comes next for clinicians and patients live at AAD 2025.

The recall includes L'Oréal's Effaclar Duo product. Stay tuned for more updates throughout the day.
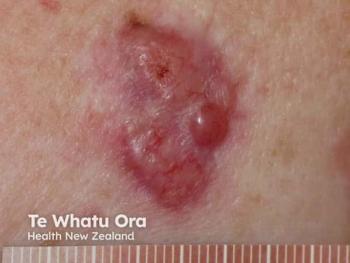
Medicus’ previous phase 1 study confirmed D-MNA’s safety, with some participants achieving complete BCC clearance.

Shawn Kwatra, MD, FAAD, reviews his late-breaking data presentation at AAD on the first original studies of a topical cream for prurigo nodularis.

The company showcased advancements in tirbanibulin and its early-stage antibody LAD191.

Published in JID, the study analyzes data from the FDA’s FAERS database to examine cases of neoplasms—including skin and breast cancers—potentially associated with benzoyl peroxide use.

The findings of the MVOR-1 and MVOR-2 studies are published in JAMA Dermatology, supporting the rosacea drug’s recent FDA approval.


After just 2 weeks of twice-daily use, a pediatric patient saw visually improved skin with no adverse events.

DELTA China follows the success of European and Canadian trials, reinforcing delgocitinib’s potential as a global treatment for CHE.

A PDUFA target action date has been set for October 13, 2025.

Snail slime contains bioactive compounds that promote skin healing, hydration, and regeneration.

The POETYK PSO LTE trial found deucravacitinib effective in sustaining PASI 75 and PASI 90 responses.

Alphyn’s therapy uniquely treats both the immune system and bacterial components of atopic dermatitis.

Barbieri et al evaluated product use, benzoyl peroxide concentration, days until expiration, and more.

Nektar Therapeutics’ investigational biologic targets the IL-2 receptor complex.

Many patients, especially parents of pediatric patients, exhibit steroid phobia, impacting treatment adherence.

Long-term safety data suggests that JAK inhibitors have a lower risk of cardiovascular and thromboembolic events than previously thought.

The drug, a JAK inhibitor, met all primary and secondary endpoints in a phase 3 trial for adolescent CHE.
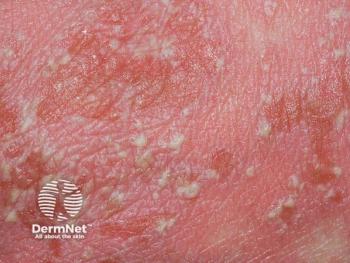
The IL-36R antagonist has demonstrated its safety and efficacy for generalized pustular psoriasis in GEMINI-1 and GEMINI-2 studies.