
Merck and Eisai discontinued the trial based on recommendations of an independent data monitoring committee.

Merck and Eisai discontinued the trial based on recommendations of an independent data monitoring committee.

Verrica Pharmaceuticals is developing a potentially first-in-class oncolytic peptide.

Three major NDAs and sBLAs were accepted by the FDA in Q1 of 2023, with PDUFA dates set in 2023 and 2024.

Late-breaking positive phase 2a results were presented at AAD 2023.

The selective and reversible Janus kinase inhibitor is being studied in several immune-mediated inflammatory diseases.

Incyte’s MCC indication is approved under accelerated approval based on tumor response rate and duration of response.

Timber Pharmaceuticals is on its way to developing the first FDA-approved treatment for congenital ichthyosis subtypes.
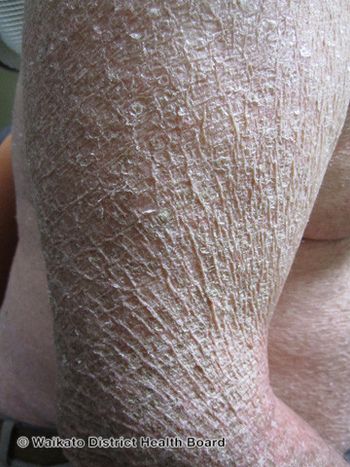
Yale School of Medicine is a testing site for a potential new congenital ichthyosis treatment.

Novan’s expected PDUFA date is January 5, 2024.

JNJ-2113 met primary endpoints in the FRONTIER 1 clinical trial.

VP-102 could be the first FDA-approved treatment for molluscum contagiosum.

Nivolumab for completely resected stage IIB or IIC melanoma is also validated by the European Medicines Agency.

Biosimilars have been a trending topic in 2023 with 8 adalimumab biosimilars expected to hit the market this year.

Adolescents and adults with seborrheic dermatitis may have a new topical treatment soon.

The expanded approval is based on data from a recent phase 3 clinical trial.

Chronic hand eczema can have a significant negative impact on quality of life.

Newly released safety and efficacy data on Novartis’ secukinumab for hidradenitis suppurativa is published in The Lancet.

Christopher Bunick, MD, PhD, and Raj Chovatiya, MD, PhD, discuss the benefits of adalimumab-atto and the future of additional biosimilars in 2023.

Preventing generalized pustular psoriasis may be possible based on recent data.

Breakthroughs and approvals we can expect in the coming year.

Potential FDA approval is expected in the first quarter of 2024.

Arcutis Biotherapeutics' indication expansion is based on data from MUSE studies.

Dermavant announced positive results from its pediatric maximal usage study.

BLA submission is based on phase 3 trial results evaluating the safety and efficacy of lebrikizumab.

Brexogen Inc.’s exosome therapy is the first entry of a clinical trial of exosome treatment for atopic dermatitis.