
The FDA has approved a single-dose pre-filled pen for dupilumab (Dupixent, Sanofi and Regeneron) for at-home administration.

The FDA has approved a single-dose pre-filled pen for dupilumab (Dupixent, Sanofi and Regeneron) for at-home administration.

Mallinckrodt announces the FDA approval of StrataGraft (allogeneic cultured keratinocytes and dermal fibroblasts in murine collagen – dsat) for the treatment of adults with thermal burns.

The B-SIMPLE4 phase 3 trial achieved positive efficacy results for the treatment of molluscum contagiosum.

John Browning, MD, FAAD, FAAP, MBA, discusses the impact of the recent FDA approval of secukinumab (Cosentyx, Novartis) for the treatment of moderate to severe plaque psoriasis in children and adolescents.

Emma Guttman-Yassky, MD, sits down with Dermatology Times® to discuss recently published results of a phase 3 study evaluating upadacitinib (Rinvoq, AbbVie) as a potential treatment of atopic dermatitis.

Revance provides an update on the approval process for its injectable daxibotulinumtoxinA (DAXI) for the treatment of glabellar lines.
Dermavant has submitted a New Drug Application (NDA) to the FDA seeking approval for its novel topical for the treatment of mild, moderate, and severe plaque psoriasis in adult patients.
The anti–PD-L1, fully human monoclonal antibody cosibelimab will be examined in a cohort of patients with metastatic cutaneous squamous cell carcinoma (cSCC) as part of an ongoing phase 1 study.

Ruxolitinib cream (Jakafi, Incyte) demonstrated positive results in 2 phase 3 studies investigating the topical for treatment of vitiligo in patients 12 years and older

Novel biologics are moving into off-label indications to expand the dermatologic armamentarium for a broad range of inflammatory skin diseases.

The FDA has granted an Orphan Drug designation to the investigational tumor-infiltrating lymphocyte therapy ITIL-168 for the treatment of patients with stage IIB to IV melanoma.
Voltaire-X study data shows that switching several times between Cyltezo and Humira results in similar pharmacokinetics, efficacy, immunogenicity, and safety in people with moderate to severe chronic plaque psoriasis.

Phase 3 trial of Otezla shows improved measures of disease severity in adults with mild to moderate plaque psoriasis regardless of their Body Surface Area affected by the disease.

Nemolizumab shows positive results according to a recently published post hoc analysis of a phase 2b trial evaluating the drug vs placebo in adult patients with moderate to severe atopic dermatitis (AD).

Single-agent pembrolizumab yielded robust antitumor activity in patients with locally advanced or recurrent/metastatic cutaneous squamous cell carcinoma.
A study finds low risk of malignancy from secukinumab treatment for psoriasis, psoriatic arthritis and ankylosing spondylitis patients, despite limited data.
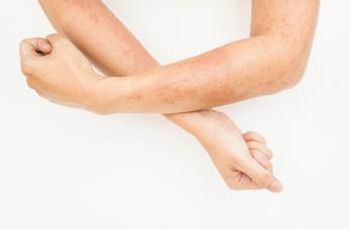
Initial rates of common treatment-emergent adverse events from baricitinib 2 mg appear to remain stable or decrease with prolonged exposure.

Timber Pharmaceuticals's phase 2b trial of isotretinoin for congenital ichthyosis (CI) has reached 50% enrollment.
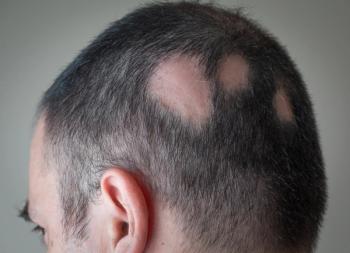
Eli Lilly and Incyte announce results from its phase 3 study evaluating baricitinib 2 mg and 4 mg for the treatment of alopecia areata, making it the first Phase 3 study with top-line results in patients with the hair disorder.
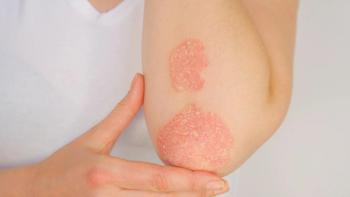
AbbVie announces the submission of regulatory filings for risankizumab to the FDA and EMA seeking approval for the drug as a treatment for psoriatic arthritis.

Explore the dermatologic drugs the FDA will make a decision to approve during April 2021.

The FDA has approved cemiplimab-rwlc as the first immunotherapy for use in patients with advanced basal cell carcinoma that has previously been treated with a hedgehog pathway inhibitor (HHI) or for whom a HHI is not appropriate.
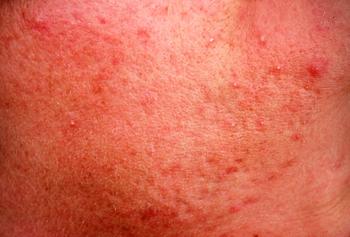
ZILXI (FMX103, VYNE Therapeutics), a 1.5% topical minocycline foam, is now available for prescription for the treatment of inflammatory lesions of rosacea in adult patients.

UCB announces the U.S. Food and Drug Administration and the European Medicines Agency have accepted the Biologics License Application and Marketing Authorization Application for bimekizumab for the treatment of moderate-to-severe plaque psoriasis in adults.

Merck announces positive results from its phase 2b trial evaluating sonelokinab, an investigational anti-IL-17 A/F nanobody, in adult patients with moderate-to-severe chronic plaque psoriasis.