
Galderma’s nemolizumab is the first approved monoclonal antibody specifically inhibiting the signaling of IL-31.

Galderma’s nemolizumab is the first approved monoclonal antibody specifically inhibiting the signaling of IL-31.
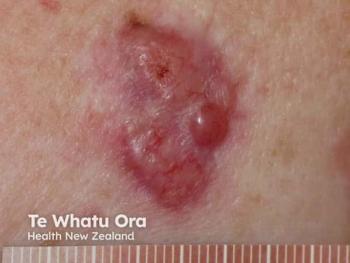
To improve overall outcomes for patients with large basal cell carcinomas, it has been beneficial to employ the use of vismodegib as a neoadjuvant and/or adjuvant treatment.

A recent Australian study reviewed appropriate terminology, gender affirming options, and dermatological considerations in caring for gender-diverse patients.

This week’s collection of the latest dermatologic studies includes the impact of isotretinoin treatment on femoral cartilage thickness, cystic fibrosis dermatitis arthritis syndrome, the role of intratumoral therapies for cutaneous melanoma, and fractional pigment toning for treating benign epidermal pigmented lesions.

Jared Gollob, MD, shares highlights of Kymera Therapeutics' recent JID publication.

Sachin Shridharani, MD, FACS, discusses the READY-1 and READY-2 clinical trials and positive support for Relfydess.

Kristine Kucera, MPAS, PA-C, DHS, discusses her patient's challenging atopic dermatitis that was eventually cleared with upadacitinib.

Keep up with the latest headlines in dermatology from the past week, including the FDA's approval of Lymphir for CTCL, L'Oreal buying stake in Galderma, and more.

In case you missed it, this week we had news about the FDA approval of denileukin diftitox for relapsed or refractory cutaneous T-cell lymphoma, the initiated phase 3 trial of ESK-001 for plaque psoriasis, secukinumab for HS clusters, and more.

Researchers linked higher exposure to natural environments and green spaces with an increased risk of developing psoriasis.

Explore how surveyed providers perceive current and emerging therapies in the atopic dermatitis.

Researchers emphasized the need for improved reliability and regulatory frameworks for AI tools before they are integrated into dermatological practice.

Secukinumab improved outcomes in all clusters, with more frequent dosing benefiting severe cases.

Melasma, challenging due to varied pigmentation in different skin types, requires personalized, tailored care.
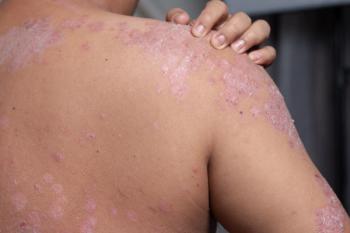
Increased COPD prevalence in patients with psoriasis likely reflects smoking correlation, according to researchers.
ND-ZnO technology also demonstrated substantial skin permeation and active ingredient delivery.

Rocco Serrao, MD, FAAD, reviewed a case study of a 20-year-old man with segmental vitiligo and the psychological impact of his vitiligo.
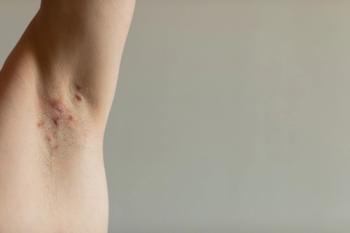
Follow-up results showed that 6 out of 9 patients achieved HiSCR at 3 months, with 5 maintaining this response at 6 months.

The approval is supported by positive phase 3 data for Lymphir in this indication.

Young patients are heading back to school, and common skin issues like acne, eczema, warts, and pigmentation can affect their confidence, Shanna Miranti, MPAS, PA-C, writes.
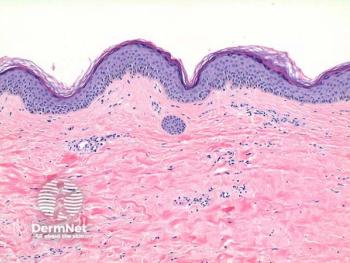
Topical corticosteroids are the gold standard treatment for VLS, yet women with skin of color are not represented in studies.
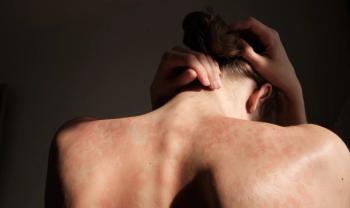
Both 8-week and 16-week dosing intervals of guselkumab achieved similar clinical outcomes and patient-reported responses in the GUIDE trial.

Researchers said they hope this consensus guides prescribing decisions among clinicians and encourages development of a standard approach.

Our August issue covers common back-to-school skin conditions, psoriasis research, OX40 inhibition for atopic dermatitis, and pediatric dermatology meeting highlights.

A group of international experts focused on the technology’s potential to standardize and enhance patient assessments.

The findings highlight the need for diverse genetic studies to understand their broader impact on autoimmune diseases.
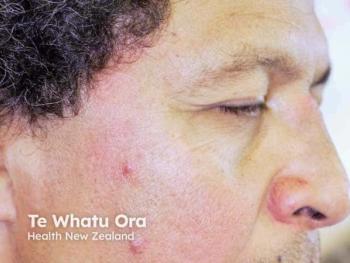
Recent research evaluated 6 patients with rosacea treated with JAK1 inhibitors upadacitinib and abrocitinib.

The 15-piece questionnaire is designed to be quick and easy to use during consultations, with particular benefits across numerous specialties.

Alumis' Martin Babler shared insights into the program for the TYK2 inhibitor and the company's next steps for its development.
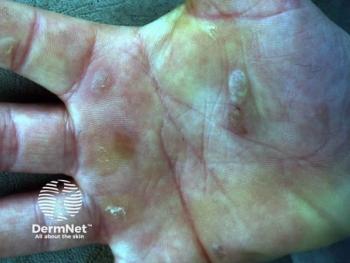
A new method using 2 single-guide RNA/Cas9 tools to target 3 specific COL7A1 gene segments with harmful mutations successfully removed up to 95% of those segments.