
Alembic Pharmaceuticals announces the launch of their bioequivalent and therapeutically equivalent product to Differin Gel 0.3% (Galderma), Adapalene Gel USP 0.3%, a topical retinoid for the treatment of acne vulgaris in patients 12 years and older.

Alembic Pharmaceuticals announces the launch of their bioequivalent and therapeutically equivalent product to Differin Gel 0.3% (Galderma), Adapalene Gel USP 0.3%, a topical retinoid for the treatment of acne vulgaris in patients 12 years and older.

Clascoterone cream 1% (Winlevi, Cassiopea), a first-in-class androgen receptor inhibitor, has been approved by the U.S. FDA for treatment of acne vulgaris in patients 12 years and older.

Will the current practice of using telemedicine for acne care have an impact on the future of dermatology? We spoke with Julie Harper, M.D., owner and president of the Dermatology and Skin Cancer Center of Birmingham in Alabama, on the topic.

Virtual visits may allow providers to focus more on patient education, says Julie Harper, M.D., owner and president of the Dermatology and Skin Cancer Center of Birmingham in Alabama.

Combination therapies that integrate older therapies have shown great promise in the treatment and management of keloids.

Julie Harper, M.D., owner and president of the Dermatology and Skin Cancer Center of Birmingham in Alabama, discusses how she approaches treating acne via virtual visits after incorporating telemedicine into her practice due to the COVID-19 pandemic.

Smartphones may provide another tool for physicians to assess acne severity in patients with darker skin types, a study shows.

Is telemedicine a useful tool to treat acne during the COVID-19 pandemic? Julie Harper, M.D., owner and president of the Dermatology and Skin Cancer Center of Birmingham in Alabama, shares her insights.

Julie Harper, M.D., owner and president of the Dermatology and Skin Cancer Center of Birmingham in Alabama, emphasizes the importance of ensuring timely treatment of acne patients during the COVID-19 crisis.

The implementation of artificial intelligence technology in the long-term management and follow-up of acne patients may help to improve patient treatment outcomes.

While recognizing that a patient’s mental health generally improves with isotretinoin use due to improvement in acne symptoms, clinicians may consider screening for insomnia during the course of treatment, as it may be an indicator for vulnerability to side effects, researchers say.

Many current acne clinical trials do not consider those patients already on hormone therapy, which researchers say may affect therapeutic outcomes. Authors of a recent study encourage this reporting in future studies to help clinicians better assess how the data might represent a practice population.
A recent Cochrane review compared the benefits of several topical acne treatments. Researchers found mixed quality of evidence for PGA outcomes. In the absence of high-quality evidence, management decisions should be guided by clinical experiences and patient preferences.
Almirall announces the U.S. Food and Drug Administration has approved the updated label for their oral antibiotic sarecycline (Seysara) for treatment of acne vulgaris to help promote the appropriate use of the drug to aid in preventing antimicrobial resistance commonly warned when using antibiotics
The U.S. Food and Drug Administration has approved the Abbreviated New Drug Application for topical retinoid adapalene gel USP, 0.3% from Alembic Pharmaceuticals and its joint venture Aleor Dermaceuticals for treatment of acne vulgaris.
Tazarotene lotion 0.045% (Arazlo, Ortho Dermatologics) is officially launched in the U.S. following approval by the U.S. Food and Drug Administration in late 2019 for the treatment of acne vulgaris in patients 9 years and older.

In the last three to five years, there has been a tremendous increase in interest in prescription treatments for acne and rosacea, resulting in many promising products entering the marketplace for these conditions.
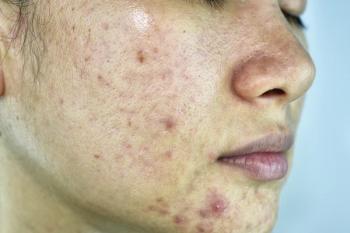
The popularity of laser and light devices has recently grown among acne patients, not only due to their e fficacy but also because treatments are quick and clean, says Michael H. Gold, M.D.
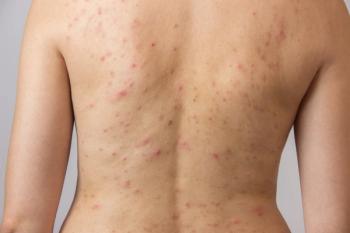
The topical combination of tretinoin 0.05% lotion and azelaic acid 15% foam improved truncal acne and may be an alternative to oral antibiotic treatment, according to recent case studies.

In honor of June being Acne Awareness Month, Thayers has launched a new line of acne-tailored skincare products.

The authors of a recently published commentary report they’ve developed a glycolic and salicylic acid combination serum they say could disrupt traditional use of topical benzoyl peroxide and antibiotics to treat acne.

A recent clinical review proves trifarotene 0.005% cream is an effective and safe treatment for acne vulgaris on the face and neck in patients 9 years and older. Currently, the drug is one of four topical retinoid acne treatments available on the market.


Menlo Therapeutics announces its wholly-owned subsidiary Foamix Pharmaceuticals Ltd. is entering a licensing with Cutia Therapeutics for the company’s FDA-approved 4% topical minocycline foam (AMZEEQ, Foamix) for acne vulgaris in pediatric and adult patients.
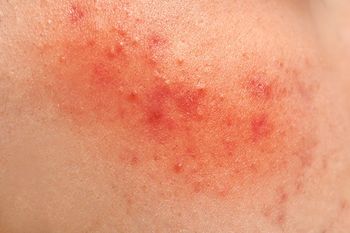
Speaking about device-based acne treatments at Maui Derm for Dermatologists 2020 meeting, Fernanda Sakamoto, Ph.D., M.D., discussed PDT and other approaches for targeting the sebaceous gland.