
In this week’s Pointers with Dr Portela, the 208SkinDoc and Cassandra Bankson, a medical aesthetician and skin advocate, react to cystic acne TikToks and offer commentary.

In this week’s Pointers with Dr Portela, the 208SkinDoc and Cassandra Bankson, a medical aesthetician and skin advocate, react to cystic acne TikToks and offer commentary.

At the 2021 Skin of Color Update, Andrew Alexis, MD, MPH, presented on the subtle distinctions in treating skin of color patients with acne.

Click here to answer our weekly poll.

This week’s Pointers With Dr Portela, Dustin Portela, DO, explains pimple patches and hydrocolloid patches, diving into how to treat acne when it surfaces.

Testosterone therapy often leads to unique challenges.

A review article investigated the psychological effects acne has on adolescent patients.

A survey conducted by Acne Wipeout and OnePoll found that Americans miss out on dating, school, hanging out with friends, and work meetings because of their skin.

A recent study investigated the difference between skin microbiota in patients with acne and controls without acne.

A study published in JAMA Dermatology investigated the strain acne puts on the lives of adult women.

Joshua Zeichner, MD, associate professor of dermatology and director of cosmetic & clinical research in dermatology, Mount Sinai Hospital, New York, discusses the impact of the recent FDA approval of Twyneo (Sol-Gel) for the treatment of acne.

Here are best practices in understanding scar morphology, building patient trust, and choosing treatment.

Twyneo, a tretinoin and benzoyl peroxide cream, has been approved by the FDA for the treatment of acne vulgaris in patients 9 years and older.

A study in the Journal of Drugs in Dermatology gathered a panel of skin of color experts to examine acne treatment in patients with skin of color.
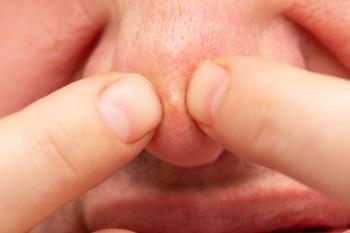
Nasal papules that are histologically indistinguishable from angiofibroma may be a sequela of acne.

IDP-126, an acne vulgaris treatment for patients ages 9 and older, met all of its endpoints in a 12-week phase 3 study.
American Academy of Dermatology’s updated guidelines on acne will cover dermatology’s hottest issues, from dosing and monitoring established medications to exploring new antibiotics and addressing antibiotic stewardship.

Emmy Graber, MD, presented the updates in acne and rosacea treatments at the SCALE Acne and Rosacea update on June 9, 2021.
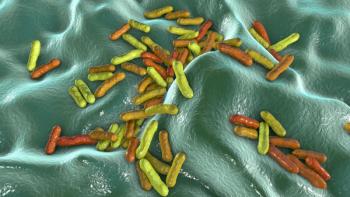
Study results reveal that nanosecond pulsed electric field therapy alters the C. acnes biofilm to enable better treatment penetration.

More than 90% of respondents to a recent survey expressed concern about acne’s effect on their adolescent’s mental health and social life.

Creating an effective treatment regimen for adult females with acne requires a holistic approach that considers the physical and psychological aspects of the world’s most common skin condition.

The SaltFacial achieves promising results in treating multiple skin conditions.

Dermatologists are divided over concerns of spironolactone being underused in women and patients taking isotretinoin being overmonitored.
A study examined the difference in prescribing patterns between pediatricians and dermatologists when it came to acne treatments.

A cohort study investigated the association between MHT and acne diagnosis.

Research presented at the EADV Spring Symposium 2021 investigated the link between gut bacteria and skin inflammation.