
A new comparative study evaluated positive outcomes from patient use of a gua sha versus a facial roller over 8 weeks of treatment.
Marie Bosslett is the assistant editor of Dermatology Times® and joined the MJH Life Sciences team in October 2024. She attended Loyola University Maryland in Baltimore and earned a Bachelor of Arts in Communication with minors in marketing and writing. When she’s not writing, Marie enjoys going to concerts, reading on the beach, and spending time with friends and family.

A new comparative study evaluated positive outcomes from patient use of a gua sha versus a facial roller over 8 weeks of treatment.

In a real-world study, the combination of Cuticapil stem hair serum and minoxidil reduced shedding and improved hair density.

Aaron Farberg, MD, led a panel exploring the challenges of diagnosing and treating generalized pustular psoriasis and the promise of targeted therapies.

At RAD 2025, the DISCOVER trial showed that dupilumab monotherapy significantly improved moderate to severe AD symptoms in this patient population.

Discover the future of regenerative skincare with Skin Moderne’s groundbreaking Exosomes Regenerative Complex, a plant-derived innovation led by Dr. Frank Roesken, MD, PhD.
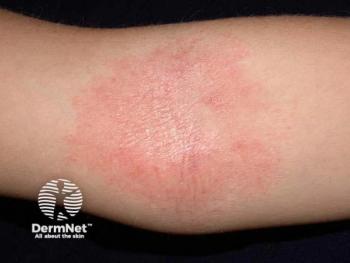
Galderma unveils promising long-term data on nemolizumab for atopic dermatitis, showcasing significant skin improvement and quality of life enhancements.

Discover the revolutionary Hydrafacial HydraFillic with Pep9 Booster, enhancing skin hydration and reducing fine lines with advanced peptide technology.

Almirall is pioneering a holistic approach in dermatology, emphasizing psychological well-being through the WHO-5 index in clinical trials.

In case you missed it, this week we had news about denifanstat's promising phase 3 results for acne, emerging therapies in lichen planus, initial insights from RAD 2025, and more.

At RAD 2025, Eli Lilly revealed promising results for lebrikizumab in treating atopic dermatitis in patients with skin of color.
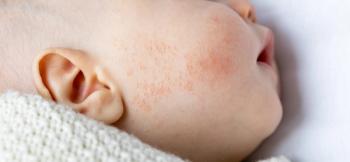
Discover groundbreaking insights from the RAD 2025 conference on roflumilast's potential to transform treatment for infant atopic dermatitis.
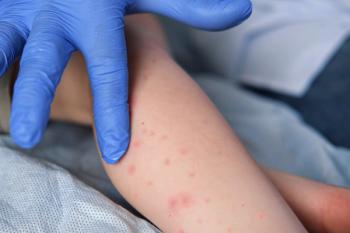
Arcutis Biotherapeutics showcases promising long-term results of Zoryve for atopic dermatitis in young children at RAD 2025, emphasizing safety and efficacy.

The OS-01 peptide formulation enhanced skin barrier function and reduced inflammation, showing promising results in a 12-week clinical trial.

Tristan Hasbargen, PA-C, led a hands-on suturing workshop at the Fall Clinical NP/PA Conference, enhancing skills in surgical dermatology.

The National Psoriasis Foundation Seal of Recognition program has expanded to prescription drugs, making roflumilast the first to achieve the indication.

Explore cutting-edge insights and networking opportunities at this year's Fall Clinical Dermatology Conference for PAs and NPs, focusing on dermatology education and innovative treatments.

Catch up on coverage from the last day of the 2025 Fall Clinical Dermatology Conference for PAs and NPs held in Orlando, Florida.

Heather Gates, PA-C, shares insights on the importance of continuous learning, mentorship, and legislative reform for PAs and NPs in dermatology.

Evan Rieder, MD, shares effective strategies to enhance dermatology practice efficiency by addressing psychological aspects of patient care and setting professional boundaries.

Catch up on coverage from the third day of the 2025 Fall Clinical Dermatology Conference for PAs and NPs held in Orlando, Florida.

Discover innovative strategies for AK treatment from Darrell Rigel, MD, MS, at the latest Fall Clinical PA/NP conference.

Discover the latest advancements in aesthetics as Heather Gates, PA-C, shares innovative techniques and emerging technologies from this groundbreaking workshop.

Catch up on coverage from the second day of the 2025 Fall Clinical Dermatology Conference for PAs and NPs held in Orlando, Florida.

At this year's Fall Clinical PA/NP meeting, Darrell Rigel, MD, MS, spoke about the evolving role of GEP in managing melanoma and squamous cell carcinoma.

Catch up on coverage from the first day of the 2025 Fall Clinical Dermatology Conference for PAs and NPs held in Orlando, Florida.

In her presentation at Fall Clinical NP/PA, Heather Gates, PA-C, shared how professionals can enhance their practice using AI and social media, fostering authenticity and patient trust in a digital age.

Explore the latest insights, strategies, and treatments for patients with skin cancer as Dermatology Times looks back on Melanoma and Skin Cancer Awareness Month.

Shannon Humphrey, MD, leads a shift in aesthetic dermatology towards patient empowerment and self-care, supported by Merz Aesthetics' Pillars of Confidence study.

Amanda Caldwell, MSN, APRN-C, is fostering community and collaboration for NPs and PAs as the new president of the SDNP.

Peter Graylin shares his transformative skin cancer journey, emphasizing the importance of early detection, sun safety, and accessible dermatology programs.