
In case you missed it, this week we had news about Alphyn Biologics' first patient dosed with zabalafin hydrogel, abrocitinib for Netherton Syndrome, the ICONIC-LEAD phase 3 study, and more.
Marie Bosslett is the assistant editor of Dermatology Times® and joined the MJH Life Sciences team in October 2024. She attended Loyola University Maryland in Baltimore and earned a Bachelor of Arts in Communication with minors in marketing and writing. When she’s not writing, Marie enjoys going to concerts, reading on the beach, and spending time with friends and family.

In case you missed it, this week we had news about Alphyn Biologics' first patient dosed with zabalafin hydrogel, abrocitinib for Netherton Syndrome, the ICONIC-LEAD phase 3 study, and more.
The presentation, led by Amy Paller, MS, MD, highlighted the upcoming phase 3 SELVA study for microcystic lymphatic malformations.

The company also announced in a press release that the product received the Seal of Acceptance by the NEA.

Google search trends and TikTok view counts were used to analyze the public popularity of cosmeceutical ingredients like retinol, hyaluronic acid, and niacinamide.

For Rosacea Awareness Month, Zoe Diana Draelos, MD, spoke to Dermatology Times about skinbetter’s Mystro Active Balance Serum and its NRS Seal of Acceptance.

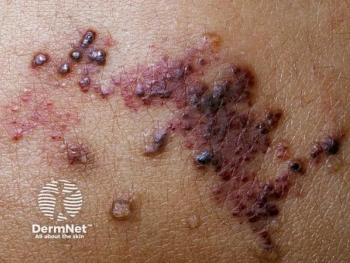
Although both methods were well tolerated, enzymatic debridement worked faster in randomized controlled trials.

Recent literature shows that children from racial minority groups with lower-income families have higher disease prevalence but reduced access to care.

The product was safe and effective after 4 weeks of topical use, specifically on patients with darker skin types.

A new case report demonstrated the JAK inhibitor’s efficacy at improving disease severity up to 6 months.
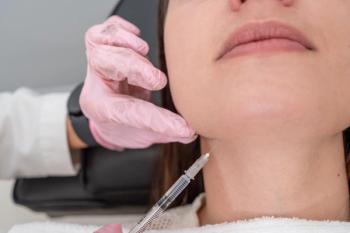
The method was safe and effective with high patient satisfaction rates 4 weeks post-injection.

In case you missed it, this week we had news about pregnancy-related skin changes, art in clinical spaces, Allergan's upcoming skin quality index, and more.

Allergan’s latest initiative hopes to empower both patients and clinicians while advancing industry standards, according to Sherket Peterson, PhD.

Sherket Peterson, PhD, spoke to Dermatology Times about Allergan’s recent research and plan to launch a universal skin quality index.

Jiyeon Oh’s research confirms that a personalized approach is needed to treat all complexities associated with AD.

Researcher Jiyeon Oh shares results of a recent study that confirm the prevalence of AD is stable, but disease burden is rising.

Positive changes in DLQI scores were observed after 4 weeks of therapy as well as the 6-month follow-up.

Misinformation from outside sources creates unrealistic expectations for pregnancy and postpartum skin conditions like hyperpigmentation.

2% and 4% formulations of the product were effective and tolerable for patients with varying scalp conditions.

Nearly all patients saw improvements in small, medium, and large depressions on the cheeks and temples with just a singular treatment session.

Compared to patients with rheumatoid arthritis and atopic dermatitis, those with vitiligo had a lower incidence of major adverse effects.

This month and all year round, we recognize the strides and contributions of female leaders in dermatology.

In case you missed it, this week we had news about CBD products in dermatology, Journey Medical's launch of Emrosi for rosacea, the psychological impact of melanoma, and more.

Patients with CHE saw higher treatment success and a favorable safety profile with delgocitinib 20-30 mg/g.

Two-sample Mendelian randomization analysis explored lifestyle factors such as sleep, smoking, alcohol consumption, and sedentary behavior.

The therapy, which combines clindamycin phosphate, adapalene, and benzoyl peroxide, was effective for Hispanic patients with moderate to severe lesions.

In a prospective study, patients with scalp seborrheic dermatitis saw positive results in efficacy, safety, and quality of life when using the AgNP product.

Treatment with tildrakizumab was still safe and effective after 1 year with notable improvements in patients’ quality of life.
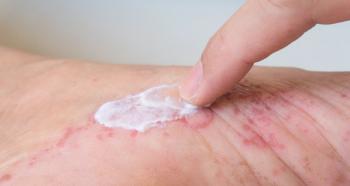
Use of the product showed positive changes in the skin’s hydration, lipid content, transepidermal water loss, and erythema.

When compared to a non-active product, the formula improved texture, reduced erythema, and strengthened the skin barrier.

The novel treatments will be for patients with orphan conditions like Netherton Syndrome, Peeling Skin Syndrome, SAM Syndrome, and palmoplantar keratoderma.