
In a clinical trial, patients reported improved quality of life after 12 weeks on sonelokimab.

In a clinical trial, patients reported improved quality of life after 12 weeks on sonelokimab.

The OSIRIS open-label study resulted in a reduction of pain and improvement in quality of life for participants.

Jane Mast, PhD, DMSc, MPAS, discusses empathetic care in hidradenitis suppurativa and the importance of supporting patients with HS in every step of their journey with the condition.

Joslyn Kirby, MD, discusses the importance of awareness and recognition in hidradenitis suppurativa from a clinician and patient perspective.

With bimekizumab, the conservative imputation method led to higher response rates, achieved endpoints, and blocked IL-17A and IL-17F.

This Hidradenitis Suppurativa Awareness Week, the Dermatology Times® team is spotlighting the often misunderstood condition and providing clinicians with the latest information in treatment and research.

This week is Hidradenitis Suppurativa Awareness Week.

This week is Hidradenitis Suppurativa Awareness Week.

This week is Hidradenitis Suppurativa Awareness Week.

This week is Hidradenitis Suppurativa Awareness Week.
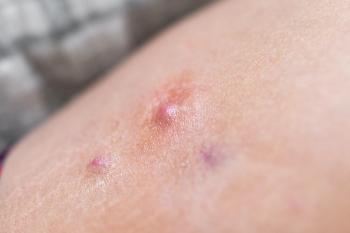
The approval makes the drug Europe’s first and only IL-17A inhibitor approved for the condition.
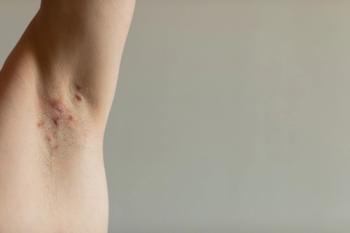
A recent study supports a degree of correlation between trough serum concentration of infliximab and clinical response to infliximab in patients with the condition.

A pair of phase 3 trials met all primary and secondary endpoints after 48 weeks.

Promising phase 2 data for LY3041658 released during the Late-breaking Research session at the 2023 annual meeting.

Expert insights on the latest issues in wounds as well as HS resources are shared at the annual meeting.

Researchers say disease severity, site, and comorbidities may lower a patient’s quality of life as they navigate hidradenitis suppurativa.

Following a systematic review and observational study meta-analysis, researchers have uncovered an association between hidradenitis suppurativa and several atopic diseases.

From topical antibiotics like clindamycin to biologic injectables like adalimumab to surgical options like deroofing, treatment for HS can come in many forms.

Newly released safety and efficacy data on Novartis’ secukinumab for hidradenitis suppurativa is published in The Lancet.

This is the first phase 3 evidence to suggest the drug as a promising treatment approach in adult patients.
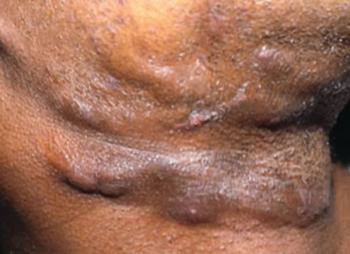
Hidradenitis suppurativa is a chronic inflammatory skin condition that presents with painful, deep, and inflamed lesions found in flexural, apocrine gland-bearing sites.
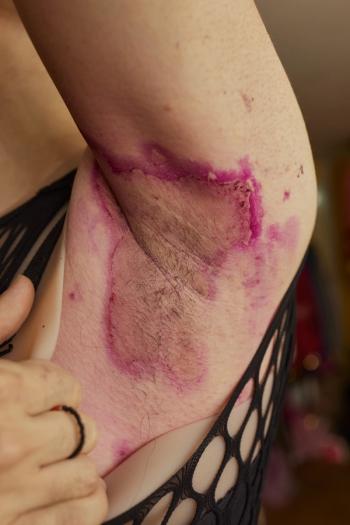
At the 2022 Fall Clinical Dermatology Conference, Joslyn R. Sciacca Kirby, MD, MS, MEd, and Raj Chovatiya, MD, PhD, discussed how there is a large unmet need for hidradenitis suppurativa treatment and what future treatment could look like.

SUNSHINE and SUNRISE trials showed superior efficacy over the placebo.

Study data was presented at EADV 2022 regarding socioeconomic status related to HS.

AnaptysBio will refocus R&D efforts on its immune cell modulator pipeline.