
Alexandra Kimball, MD, FAAD, reviews late-breaking data at AAD on lutikizumab for hidradenitis suppurativa.

Alexandra Kimball, MD, FAAD, reviews late-breaking data at AAD on lutikizumab for hidradenitis suppurativa.

How can you improve excision care for your patients with hidradenitis suppurativa? Ashley Nicole Elsensohn, MD, MPH, FAAD, shared clinical pearls at the AAD Annual Meeting.

Findings regarding an association between HS severity and dietary elements such as vitamin D, B12 supplementation, and more, are inconclusive.

Researchers from the University of Michigan and Almirall found that the profibrotic fibroblast response in HS can be modulated through inhibition of the Hippo pathway.

IV antibiotics should play a complementary role in the management of HS as new immunomodulator therapies are developed, researchers concluded.
To gauge the impact and evolving landscape of HS treatment post-secukinumab approval, Spherix Global Insights conducted research with more than 100 US dermatologists.
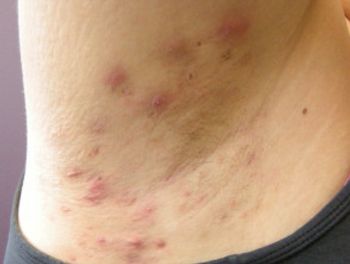
Higher response rates were observed in patients treated with lutikizumab. The drug will now advance to phase 3 clinical trials.

Results of this study may also be indicative of oral orismilast's ability to contribute to meaningful clinical improvements, investigators noted.

This CME credit opportunity delves into the pathogenesis and management of hidradenitis suppurativa.

Jared Gollob, MD, Chief Medical Officer of Kymera Therapeutics, spoke with Dermatology Times to discuss these trial results.
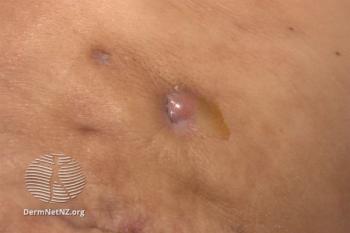
More than 80% of patients did not experience an increase in number of draining tunnels associated with HS by week 52.
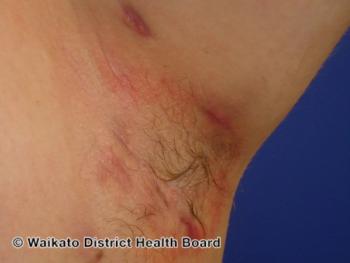
After maintenance treatment with sonelokimab dosed every 4 weeks, 57% of patients achieved HiSCR75 at week 24.
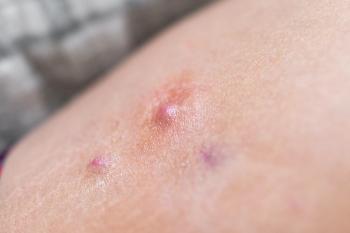
UCB presented data from the BE HEARD I and BE HEARD II phase 3 studies at this year's EADV Congress.

The addition of an HS dataset adds approximately 26,000 new patient records.

When combining adalimumab with surgery, patient outcomes, including quality of life, were improved.
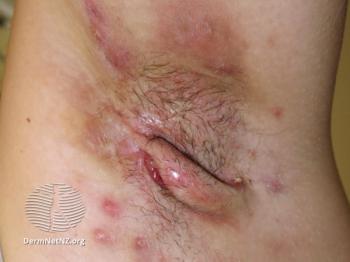
A systematic review found that generally, combination therapy has evidence supportive of its efficacy in hidradenitis suppurativa.

Changes to skin and nerve endings as a result of HS may contribute to peripheral neuropathy.

Affective, anxiety, and neurotic disorders were among the most common in a recent study.
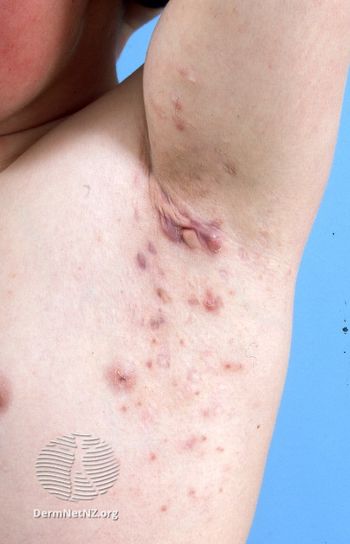
The IL-17A inhibitor did not meet its phase 2b/3 clinical trial primary end point.

The procedure was both effective and safe in a small cohort of patients with refractory hidradenitis suppurativa of the axilla.
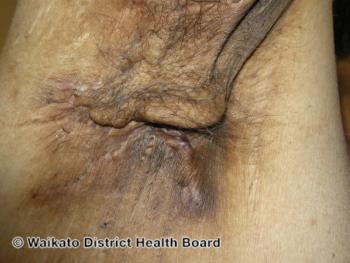
Female sex bias in hidradenitis suppurativa can be attributed to several contributing immunological factors.

Genetic variants associated with hidradenitis suppurativa (HS) were identified, although a causal effect is not proven.

Studies showed the various treatments improved symptoms, but more trials need to be done.

Researchers have isolated 2 genes, SOX9 and KLF5, that play a role in the development of hidradenitis suppurativa.

An anonymous, online survey was conducted through HS support groups from June 2022 to July 2022.