
In addition to decreases in BMI, researchers also reported fewer HS flare ups.

In addition to decreases in BMI, researchers also reported fewer HS flare ups.

This Maui Derm NP+PA Fall session discusses the latest advancements in hidradenitis suppurativa treatment, the evolving therapeutic landscape, and the critical need for timely diagnosis and aggressive care.
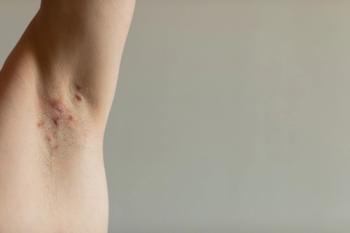
Although clinicians showed similar antibiotic prescribing rates, the study highlights a need for better adherence to HS treatment guidelines across all specialties.
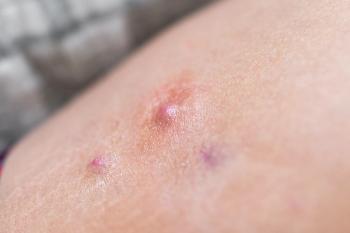
The trial revealed that 40% of patients on izokibep achieved HiSCR75 by week 16, a notable improvement over the 20% on placebo.

Jared Gollob, MD, shares highlights of Kymera Therapeutics' recent JID publication.

Secukinumab improved outcomes in all clusters, with more frequent dosing benefiting severe cases.

Follow-up results showed that 6 out of 9 patients achieved HiSCR at 3 months, with 5 maintaining this response at 6 months.
Spherix Global Insights recently delved into the efficacy, patient quality of life, and symptom management of adalimumab vs secukinumab.

Hypertension and hyperlipidemia are common among obese pediatric patients with hidradenitis suppurativa, according to a poster presented at the SPD's annual meeting.

Jennifer Hsiao, MD, shares insights on emerging therapies and comprehensive care strategies at the 2024 SDPA Annual Summer Dermatology Conference.

Henry Lim, MD, discusses key findings and areas of improvement identified by the report, as well as his hopes for HS treatment moving forward.

UCB recently announced phase 3 BE HEARD I and II trial results on bimekizumab for treating moderate to severe HS were published in The Lancet.
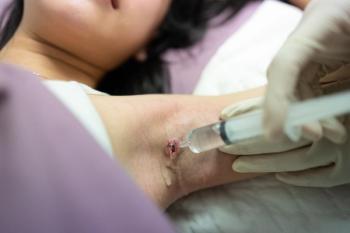
Four leaders in HS treatment and care put a spotlight on the latest clinical trial data and treatment techniques.

Hadar Lev-Tov, MD, MAS, President of the HS Foundation, discusses the organization's hopes for the future and upcoming efforts.

The Lancet data is the primary publication of bimekizumab results from BE HEARD I and BE HEARD II.
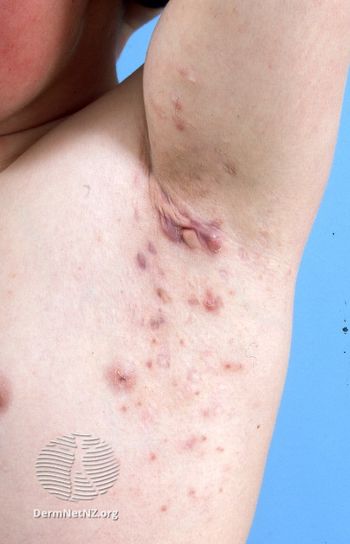
VELA is the first phase 3 hidradenitis suppurativa trial to evaluate HiSCR75 as the primary end point.

Researchers have developed a clinical decision support model using machine learning to assist health care providers in predicting diagnoses of hidradenitis suppurativa.

Adalimumab-adbm is now approved for use in various indications, including psoriasis, psoriatic arthritis, and hidradenitis suppurativa.

Earn CME credit and learn how to optimize a multimodal treatment approach for hidradenitis suppurativa.

Researchers conducted a population-based study examining a sample of patients with HS during hospital admissions.

The European approval was based on data from the Phase III BE HEARD I and BE HEARD II trials.

A study demonstrated the favorable efficacy and safety profiles of secukinumab in patients with severe HS for up to 52 weeks.
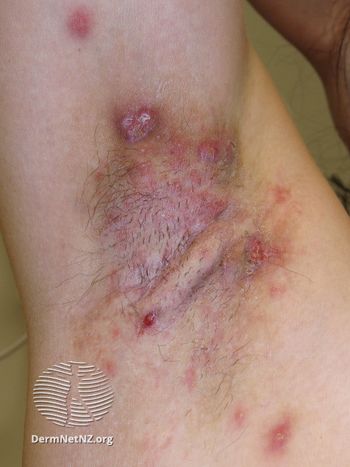
MoonLake’s sonelokimab is being evaluated for hidradenitis suppurativa, psoriasis, and psoriatic arthritis.

The submission stems from promising results from the phase 3 BE HEARD I and BE HEARD II trials.

Povorcitinib is being evaluated for non-segmental vitiligo, hidradenitis suppurativa, prurigo nodularis, asthma, and chronic spontaneous urticaria.