Hidradenitis Suppurativa
Latest News
Latest Videos

CME Content
More News

STOP-HS1 and STOP-HS2 phase 3 trials showed nearly 60% of patients achieving HiSCR50 by week 24, according to a presentation at EADV 2025.

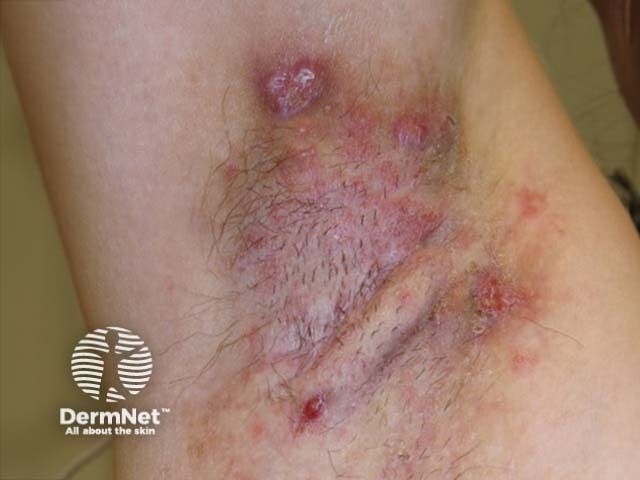
HS management is shifting toward earlier diagnosis and intervention to prevent long-term scarring and disability.

Research shows it can take 7 to 10 years for individuals with HS to receive an accurate diagnosis.

Katrina Lee, MD, discussed innovative strategies and challenges in managing HS at the Dermatology Innovation Symposium, emphasizing early intervention and comprehensive care.

A recent HS Summit hosted by UCB brought together clinicians and patient advocates to strengthen research and awareness efforts.

Andrade's inspiring journey highlights the challenges of HS and the transformative impact of clinical trials.
Steven Daveluy, MD, highlighted lack of awareness and early misdiagnosis as major barriers to effective HS care.

Our July cover story explores effective treatments for hidradenitis suppurativa, including procedural interventions and advanced therapies that enhance patient quality of life.

Both regulatory-approved and pipeline treatments for moderate to severe HS demonstrate comparable efficacy and safety, offering hope for patients often dissatisfied with current treatment options.

A global survey reveals the significant psychosocial and financial burdens of hidradenitis suppurativa, highlighting the need for improved patient care and treatment options.

At SCALE 2025, Christina Feser, DO, FAAD, FAOCD, spotlighted the rapidly expanding pipeline of diverse therapies for patients with HS.

At RAD 2025, Matthew Zirwas, MD, outlined 2 upcoming shifts in dermatology: the emergence of povorcitinib for hidradenitis suppurativa and the anticipated generic availability of tofacitinib.

Adam Friedman, MD, FAAD, discusses the growing need for psychosocial support in hidradenitis suppurativa care and HS Connect’s role in closing the gap.

Celltrion's Yuflyma gains full FDA interchangeability, enhancing patient access and affordability for various inflammatory conditions.
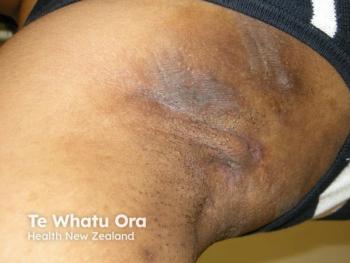
Explore the critical need for diversity in hidradenitis suppurativa clinical trials to address health inequities and improve patient outcomes.
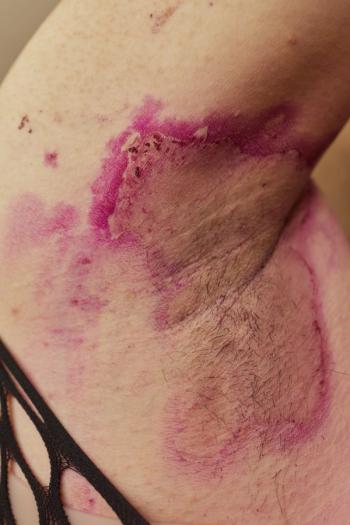
Ixekizumab shows promise in restoring clinical response for hidradenitis suppurativa patients previously unresponsive to adalimumab, highlighting new treatment options.
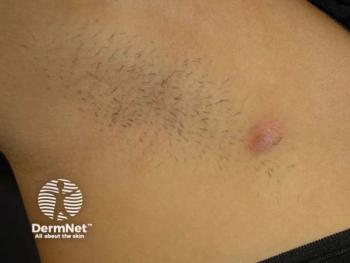
A cross-sectional survey revealed that many patients with HS are dissatisfied with treatment options, with gaps in knowledge about FDA-approved therapies.

In part 3 of this Frontline Forum supplement, experts discuss surgical innovations in HS, setting treatment expectations, and more.

In part 2 of this Frontline Forum supplement, experts discuss current and emerging treatments, digital tools and supports, and more.

In part 1 of this Frontline Forum supplement, experts discuss the burden of HS, reducing diagnostic delays, and more.
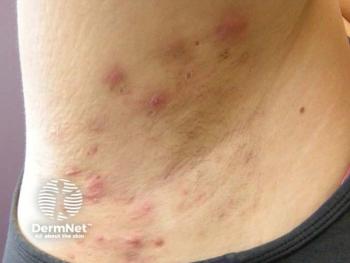
Incyte’s phase 3 STOP-HS trials demonstrated significant efficacy and safety of povorcitinib in moderate to severe hidradenitis suppurativa.

Karl Yen of Sanofi discussed the role of amlitelimab in AD following sustained results and a promising safety profile presented in data at AAD.

Novartis highlighted key findings at AAD and AAAAI, showcasing Cosentyx's flexible dosing for hidradenitis suppurativa and remibrutinib’s efficacy for chronic spontaneous urticaria.

Efficacy, safety, and pharmacokinetic data support the use of vilobelimab in treating pyoderma gangrenosum and hidradenitis suppurativa.

Stark highlighted Bimzelx’s role in treating psoriasis and HS, introduced the BE BOLD study for PsA, and looked to future innovation in inflammatory skin disease.