
Camp Wonder, a camp that supports kids with skin disease and is sponsored by Galderma, is celebrating their 20th year of operations this week.

Camp Wonder, a camp that supports kids with skin disease and is sponsored by Galderma, is celebrating their 20th year of operations this week.

Gastrointestinal infections were the most common serious infections associated with biologics, according to a cohort analysis.

Addressing underdiagnosis, underrepresentation, and undertreatment in skin of color patients with psoriasis.
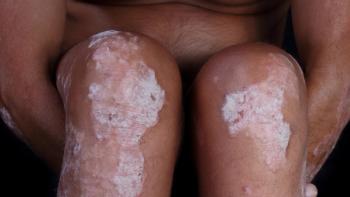
Engage patients in treatment compliance and lifestyle changes to achieve optimal outcomes.
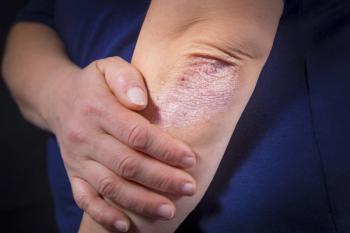
Sol-Gel has announced pre-clinical data on SGT-510, its roflumilast drug, for the treatment of psoriasis.

The FDA announces it did not meet the June 25 PDUFA action date on the sNDA for active psoriatic arthritis treatment, upadacitinib.
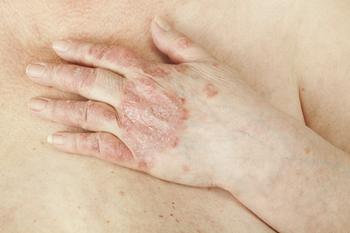
COSMOS phase 3b study data demonstrated the effectiveness of guselkumab in patients diagnosed with psoriatic arthritis.

The National Psoriasis Foundation has updated its guidance statements for individuals living with psoriatic disease during the COVID-19 pandemic.
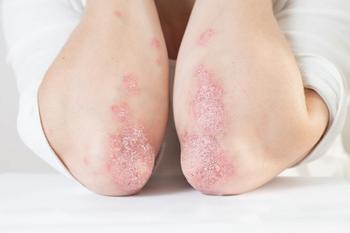
The pro-inflammatory transcription factor is critical for the expression of IL-23, which is known to play a role in psoriasis.

Biologic therapy has proven very useful in treating an increasing number of dermatologic diseases and conditions. However, caution is warranted for patients with chronic infectious diseases.

John Browning, MD, FAAD, FAAP, MBA, discusses the impact of the recent FDA approval of secukinumab (Cosentyx, Novartis) for the treatment of moderate to severe plaque psoriasis in children and adolescents.
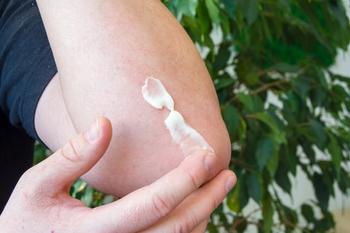
Dermavant has submitted a New Drug Application (NDA) to the FDA seeking approval for its novel topical for the treatment of mild, moderate, and severe plaque psoriasis in adult patients.

Secukinumab (Cosentyx, Novartis) has received FDA approval for the treatment of children and adolescents with moderate to severe plaque psoriasis.

AxisBiotix-Ps food supplement shows positive results in a recent consumer study evaluating its efficacy for psoriasis treatment.

Thanks to targeted drugs in early-stage development, patients with generalized pustular psoriasis (GPP) will one day likely have FDA-approved treatments for the disease, according to one expert.

In this episode, we take a look back at what happened at the American Academy of Dermatology Virtual Meeting Experience 2021 (AAD VMX 2021), including exclusive interviews with two KOLs about their presentations regarding climate change and new data on biologics for psoriasis.

Novel biologics are moving into off-label indications to expand the dermatologic armamentarium for a broad range of inflammatory skin diseases.

A new study shows a possible association between fasting and skin improvement in psoriasis patients.
Psoriasis patients may have a higher risk of contracting COVID-19, according to study results recently presented at AAD VMX 2021.

Research presented at the EADV Spring Symposium 2021 investigated the link between gut bacteria and skin inflammation.
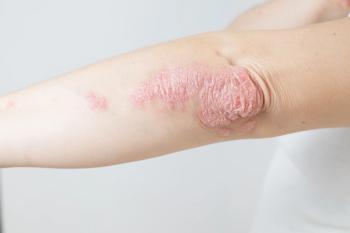
After data from a phase 3 study, FDA opens potential for apremilast to be the first and only approved oral therapy for mild to moderate plaque psoriasis.

Mark Lebwohl, MD, discusses his recent presentation from AAD VMX 2021 regarding new data for various biologics for the treatment of psoriasis.

Study findings show calcipotriene-betamethasone foam to be safe and moderately effective in skin of color patients with psoriasis but failed to meet several secondary endpoints and to demonstrate statistically significant changes.
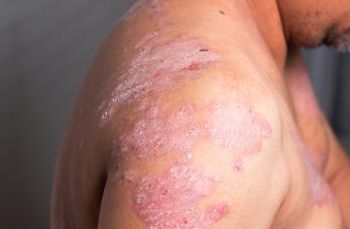
Deucravacitinib meets primary end points for treating moderate to severe psoriasis.
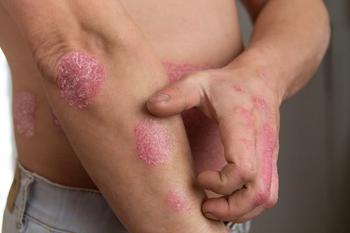
The FDA has set a Prescription Drug User Fee Act (PDUFA) date for bimekizumab (UCB) for the treatment of psoriasis.