
Factors include extent of disease, the latest findings on agents’ efficacy, and even patient weight.

Factors include extent of disease, the latest findings on agents’ efficacy, and even patient weight.
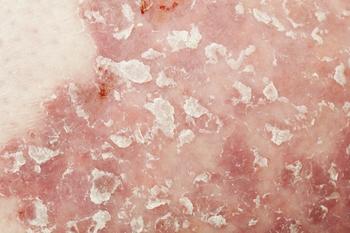
The new data examines delivery system efficacy of either 1 secukinumab 300-mg dose in an autoinjector vs 2 doses of secukinumab 150-mg 1 mL pre-filled syringes.

This episode highlights findings from an investigative study exploring the molecular targeting of biologics for psoriasis, the IL-17 drug class, how bimekizumab is different, plus more.

Even with patient hesitancy, treating PPP with gentian violet and chemical peels could help patients avoid biologics.

The FDA has approved halobetasol propionate foam, 0.05% (Lexette; Mayne Pharma), a potent topical corticosteroid, for the treatment of plaque psoriasis in adolescent patients.
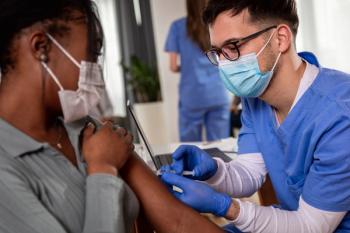
The National Psoriasis Foundation has released new guidance statements after the Centers for Disease Control and Prevention provided new information on COVID-19 vaccine boosters for immunocompromised patients.

A recently published study compared cognition, MRI-markers, and dementia risk in patients diagnosed with psoriasis vs those without psoriasis.
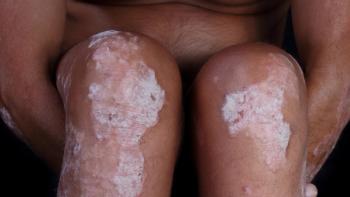
Lack of specific phenotypes complicates verification.

A study published in the Journal of Dermatological Treatment shows guselkumab may be more effective in heavier patients with moderate to severe plaque psoriasis vs secukinumab.

According to a study in Annals of Dermatology and Venerology, pediatric patients with psoriasis in France frequently missed dermatologic consultations.

Mark Lebwohl, MD, takes a deep dive in the recent delay in approval for multiple JAK inhibitor drugs for treatment of inflammatory skin conditions such as psoriasis and atopic dermatitis. He also discusses the impact of the delay, as well as the benefits of JAK inhibitors for inflammatory skin diseases, and an outlook for the future of this drug class.

The FDA is now requiring black box warning for certain JAK inhibitors used to treat arthritis. This news comes amid delay in FDA approval for multiple JAK inhibitors for the treatment of inflammatory skin conditions.

Some systemic medications may have increased risk for patients during the COVID-19 era, according to a recent Pediatric Dermatology Research Alliance (PeDRA) statement.

Theresa Vavra, PharmD, BCPS, provides studies for some of the most commonly used supplements by patients with psoriasis.

Whitney Linsenmeyer, PhD, RD, LD, provides 3 dietary considerations for patients with psoriasis.
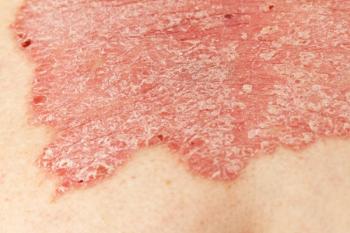
COVID-19’s lingering impact brings new considerations for treatment regimens for pediatric patients with psoriasis.

Holistic options for disease management show improvement in mild psoriasis.

UCB announced the European Commission have granted marketing authorization for bimekizumab for the treatment of moderate to severe plaque psoriasis.

Jennifer Powers, MD, associate professor of dermatology at the University of Iowa, talks her recent research investigating how diet and supplements affect patients with mild psoriasis.

In this episode, Jennifer Powers, MD, board certified dermatologist and associate professor of dermatology at the University of Iowa, discusses her recent research exploring how diet and supplements affect patients with mild psoriasis.
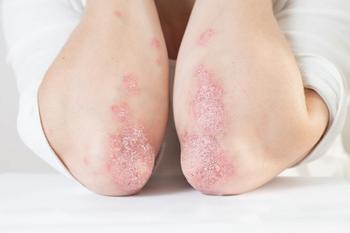
Due to the multimorbid nature of the disease, clinicians must screen, monitor, and aggressively treat the associated comorbidities.

More research is needed to understand the pathogenesis underlying this association.

The International Psoriasis Council (IPC)’s new binary classification system aims to streamline access to systemic treatments for patients who previously were likely undertreated.

UCB announced that more than 90% of patients treated with bimekizumab have maintained IGA 1/0 results in their long-term BE BRIGHT trial.

The FDA has accepted the New Drug Application (NDA) for tapinarof as a treatment for plaque psoriasis in adults and set the PDUFA target action date to Q2 2022.