Psoriasis
Latest News
Latest Videos

CME Content
More News

Drs Mark G. Lebwohl and Joseph F. Merola discuss how patient presentation differs in patients with PsO and PsA.

Mark G. Lebwohl, MD, and Joseph F. Merola, MD, MMSc, discuss updates in the pathogenesis of PsA and PsO.

Arcutis Biotherapeutics’ roflumilast cream showed higher efficacy compared to vehicle in two phase 3 studies.

At the Skin of Color Update meeting in New York City, Mona Shahriari, MD, FAAD, shares her thoughts on prescribing the recently-approved tapinarof (VTAMA) for plaque psoriasis.

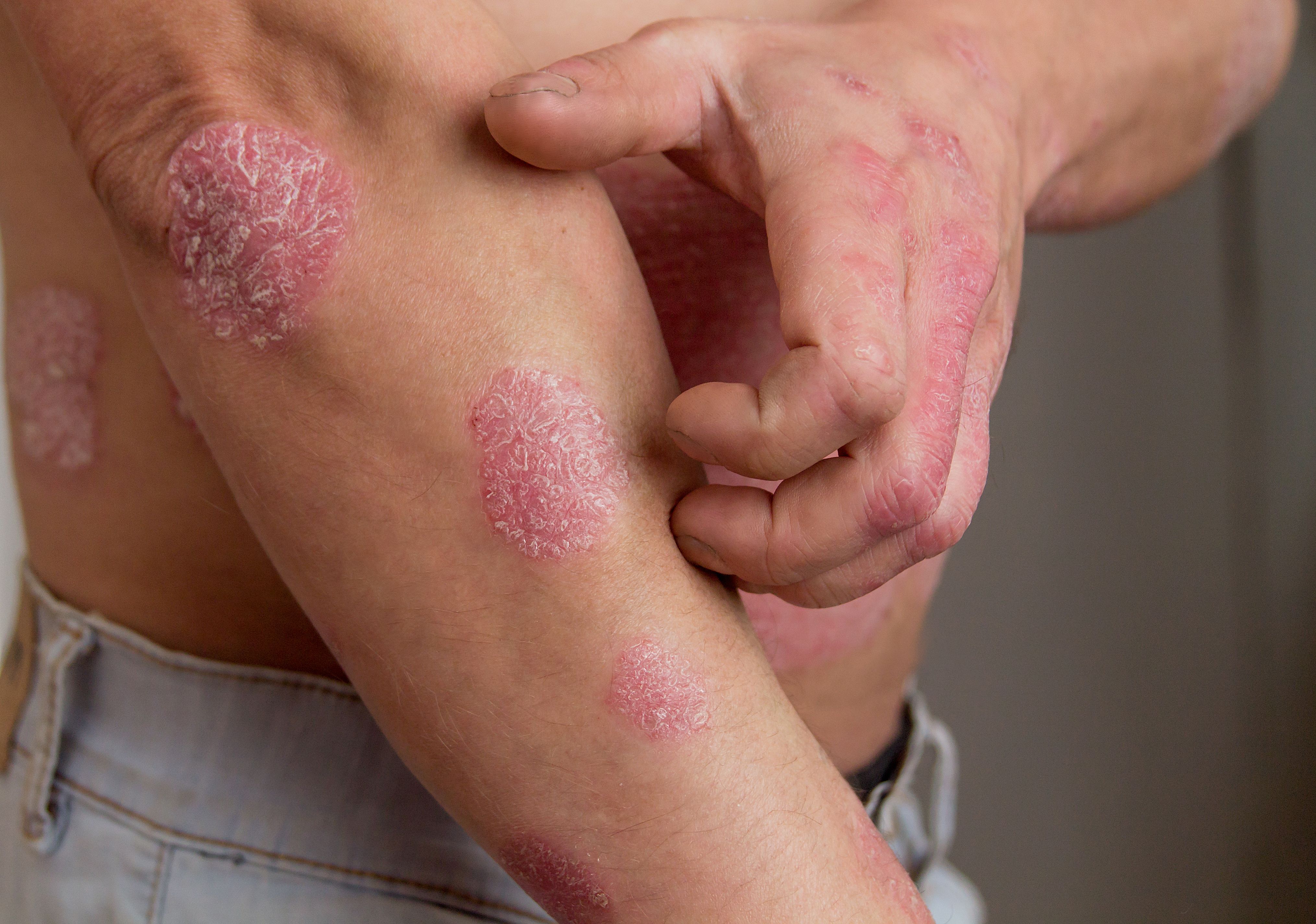
Deucravacitinib offers new hope for those with moderate-to-severe plaque psoriasis.

The relationship between mental health and psoriasis was discussed at EADV Congress 2022 in Milan, Italy.

Pediatric skin conditions can cause feelings of sadness or self-consciousness and have an impact on friendships as a result of the disease.

Wilson Liao, MD, professor and vice chair of research in the Department of Dermatology at the University of California, San Francisco (UCSF) School of Medicine, and colleagues conducted a study examining the role of supplements and diets in managing psoriasis.

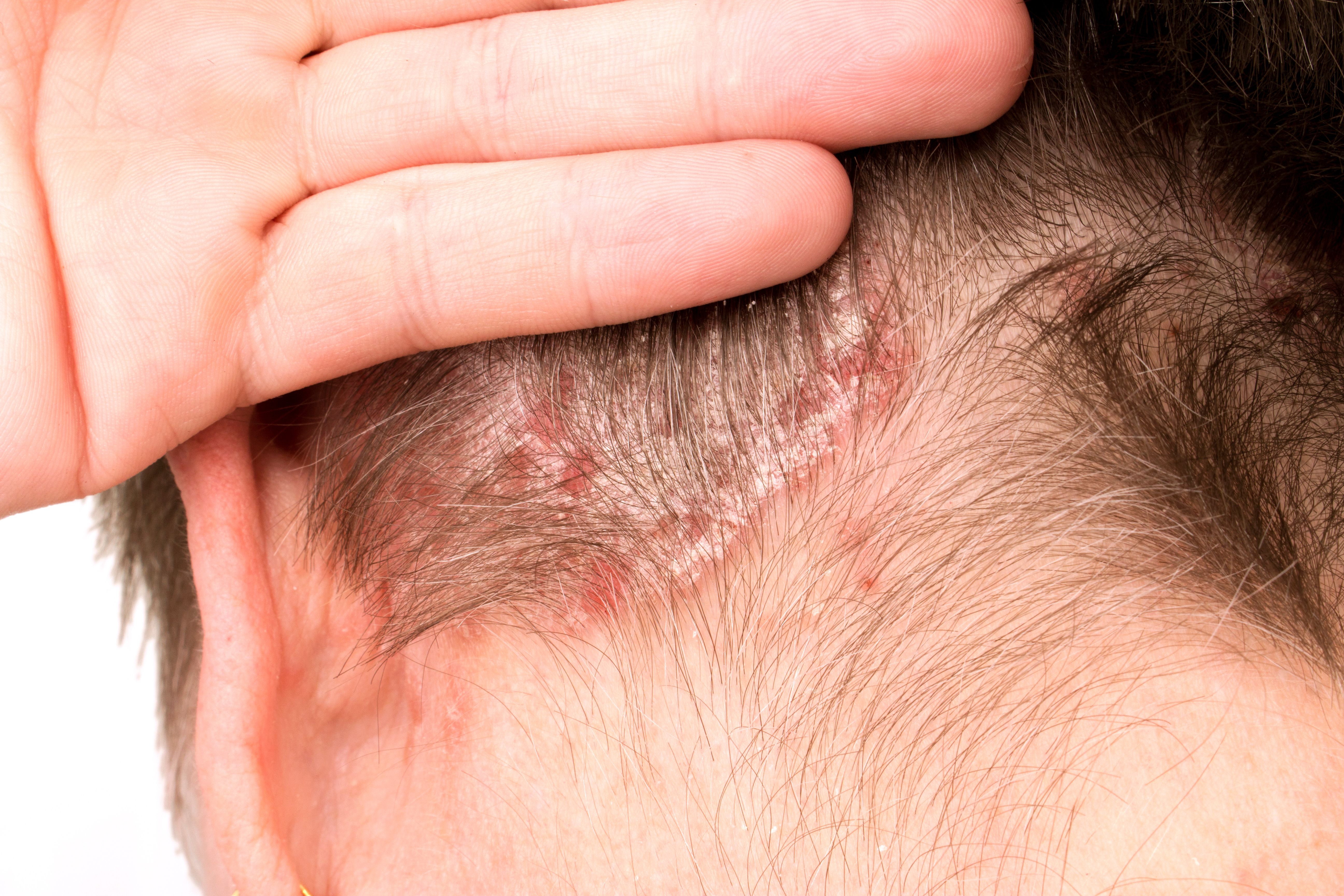
Nail psoriasis is not as uncommon as some patients with psoriasis may believe.

Results from an online, anonymous, questionnaire-based analysis conducted by Patrycja Rogowska, MD, and colleagues, were published in Clinical, Cosmetic and Investigational Dermatology.

Results of methotrexate dosing for patients with psoriasis were published in the Journal of the American Medical Association Dermatology.

Arcutis Biotherapeutics’ roflumilast cream will provide relief to patients aged 12 years or older diagnosed with plaque psoriasis.

Paller discusses the findings of the clinical trial examining the long-term effects of ixekizumab for treating psoriasis in children.

A study published in JAAD International examined the association between psoriasis and adverse pregnancy outcomes (APOs) and found that providers should be on the lookout for ectopic pregnancies (EP) in this population.

From pediatric dermatology quizzes to the latest news in telederm to practical pearls on atopic dermatitis, alopecia, and vitiligo, there’s a lot going on at the SPD 2022 47th Annual Meeting, this year in Indianapolis, Indiana.

Click here to answer this week's poll.
Arthur Kavanaugh, MD, a professor of medicine and director of the Center for Innovative Therapy (CIT) at UC San Diego in California, gives his thoughts on the treatments for psoriatic arthritis, the comorbidities that have to be considered, and more.

A new study aimed to evaluate the differences in the immunohistochemical staining (IHS) in patients with palmoplantar pustulosis and determine whether they can be divided into two distinct groups based on their histopathological profiles

A recent study dove into how psoriasis and sleep interruptions are related to each other.

At the 2022 SDPA Annual Summer Meeting, Lisa Swanson, MD, shared some fascinating patient presentations in pediatric dermatology.

At the 2022 National Infusion Center Association Annual Conference, Kelly Gordon, PharmD, answered why diagnosing GPP is so difficult and what to look for to optimize patient care.

Education, honesty, and communication skills are key for both patient and dermatologist when it comes to creating treatment regimens.

In a video with Dermatology Times®, James Q. Del Rosso, DO, discusses his presentation “What’s News in the Medicine Chest” at the current 2022 Fall Clinical Dermatology Conference for PAs & NPs.
In a hard-hitting session from the 2022 Fall Clinical Dermatology Conference for PAs & NPs, April Armstrong, MD, MPH, dove into PAD technology and future innovations in psoriasis treatment.

Dermavant’s new topical treatment for plaque psoriasis in adults was approved by the FDA this morning.