Pediatric Dermatology
Latest News
Latest Videos

CME Content
More News

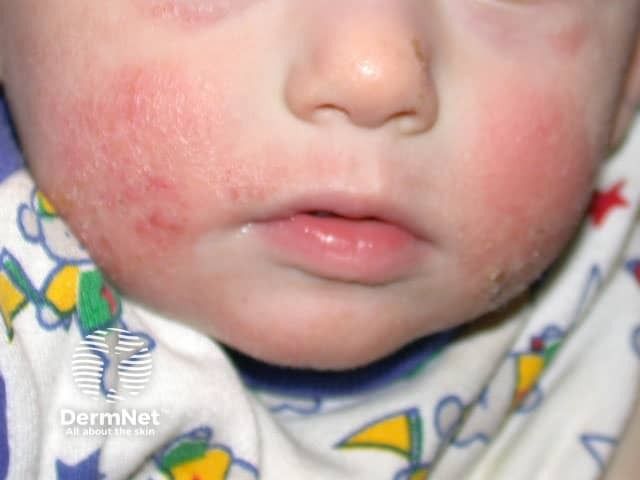
Pediatric dermatologist Mercedes E. Gonzalez, MD, discusses roflumilast's promise as a safe, nonsteroidal treatment for atopic dermatitis in infants.
Mercedes Gonzalez, MD, discusses the promising INTEGUMENT-INFANT trial, exploring a new non-steroidal treatment for infants with atopic dermatitis.

Discover insights from Peter Lio, MD, on precision medicine in pediatric atopic dermatitis and the promising role of ruxolitinib at the RAD 2025 Conference.

At RAD 2025, pediatric dermatologist Lisa Swanson, MD, and allergist Anne Marie Singh, MD, led a dual session aimed at demystifying the connection between food allergy and atopic dermatitis.
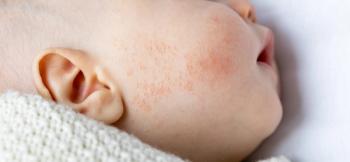
Discover groundbreaking insights from the RAD 2025 conference on roflumilast's potential to transform treatment for infant atopic dermatitis.
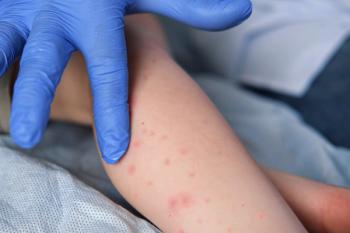
Arcutis Biotherapeutics showcases promising long-term results of Zoryve for atopic dermatitis in young children at RAD 2025, emphasizing safety and efficacy.

Peter Lio, MD, discusses innovative treatments for pediatric atopic dermatitis, including microbiome therapies and botanical options.

A new study reveals ongoing burden of moderate to severe atopic dermatitis in youth, with flares, comorbidities, and quality of life impacts.

Sophisticated colloidal oat emollients show promise in effectively managing pediatric atopic dermatitis, enhancing skin hydration and reducing flare-ups in infants.

The novel formulation was compared to a petrolatum-based diaper and was found to be just as effective for irritation and redness.
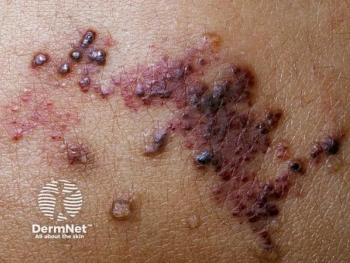
The presentation, led by Amy Paller, MS, MD, highlighted the upcoming phase 3 SELVA study for microcystic lymphatic malformations.
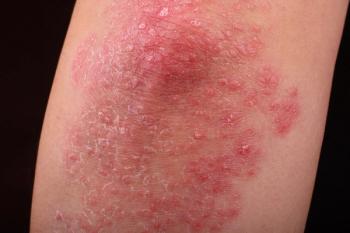
A new subgroup analysis revealed higher rates of clear or almost clear skin at week 16 vs placebo with a favorable safety profile.

Recent literature shows that children from racial minority groups with lower-income families have higher disease prevalence but reduced access to care.

The study stated early recognition and aggressive treatment of childhood-onset MMP are essential to prevent permanent scarring.

Mercedes E. Gonzalez, MD, reflects on the SPD meeting as well as challenges and advances in pediatric dermatology.

Compared to placebo, there was a 70% reduction in IgE levels in children ages 0.5 to 6 after 16 weeks of therapy.

Lisa M. Arkin, MD, shared how early laser treatment and multidisciplinary approaches improves outcomes for vascular anomalies.

Linda Stein Gold, MD, FAAD, discusses the late-breaking tapinarof cream data presented at AAD 2025.
Experts discussed pediatric atopic dermatitis, treatment innovations, disease burden, and the latest topical and systemic therapies in a DermView video series.

A PDUFA target action date has been set for October 13, 2025.

At the Horizons in Advanced Practice meeting, Lakshi Aldredge, MSN, ANP-BC, DCNP, FAANP, presented a case of a female patient aged 12 years with moderate AD that had been worsening over the past several months.

Researchers reported that propranolol initiation for infantile hemangiomas in pediatric patients is safe, with no significant complications, suggesting reduced need for intensive monitoring.
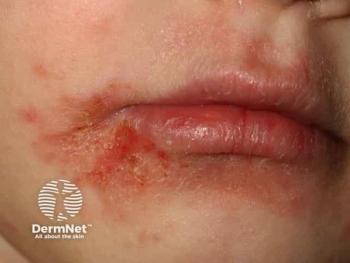
Lead author on recent research, Lawrence Eichenfield, MD, said if approved, this treatment could fill a “significant gap.”

The promotion of anti-aging products to children, who don't yet experience age-related skin changes, is both ethically questionable and potentially harmful.

Patients aged 6 to 12 had little to no adverse events and no pharmacokinetic abnormalities were noted.