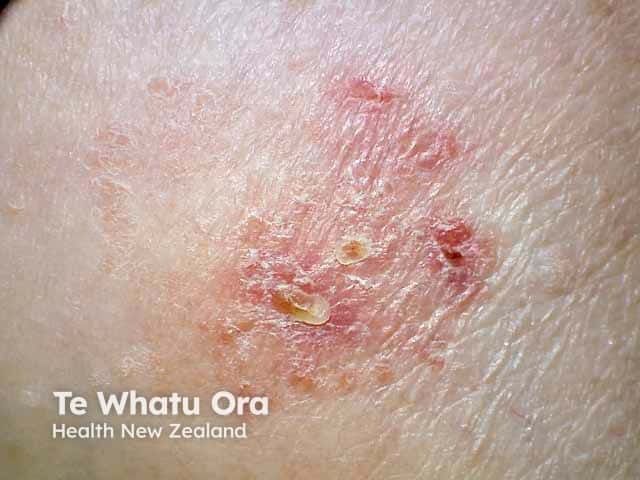
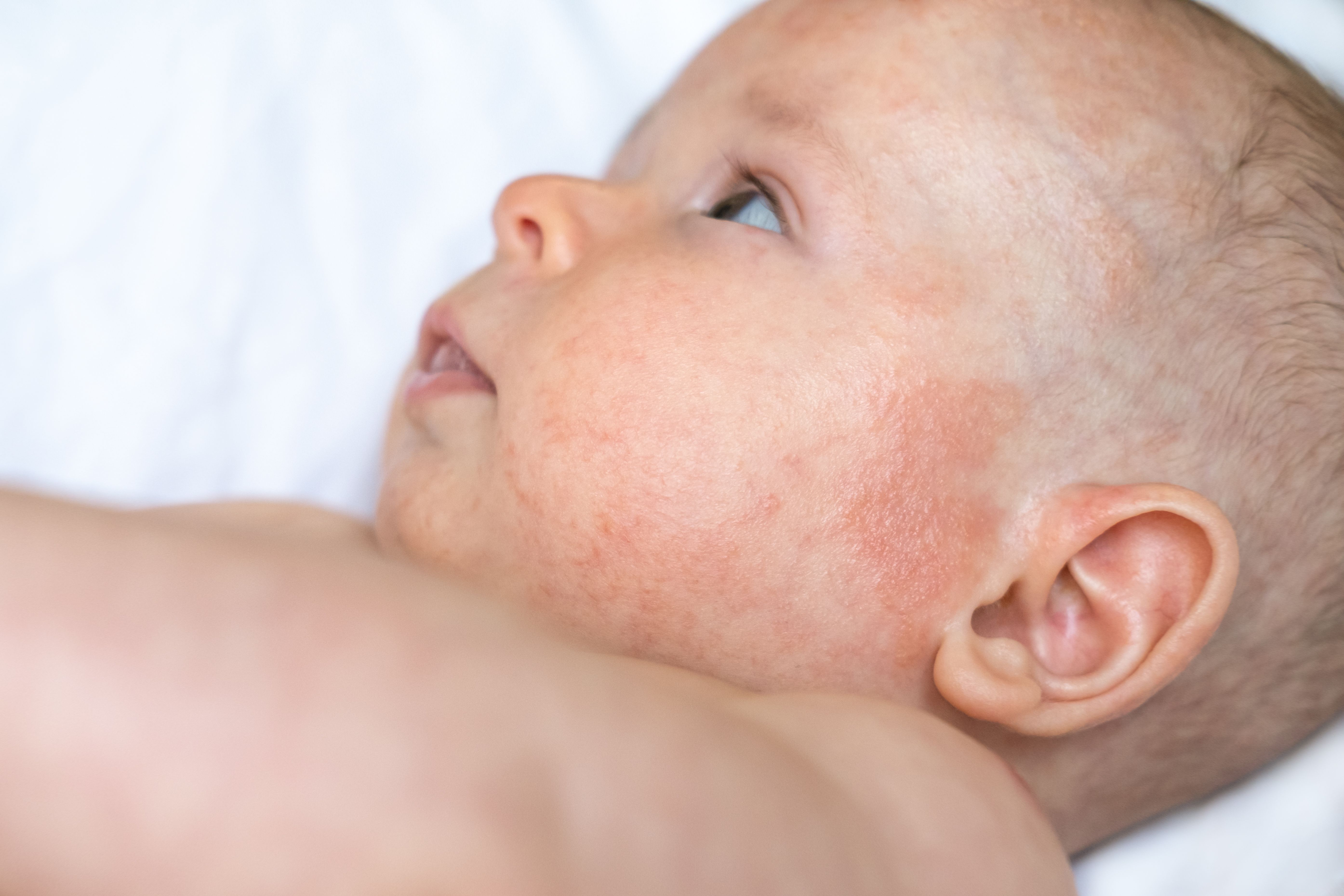
Atopic Dermatitis
Latest News

Latest Videos

CME Content
More News

The exclusive event brought together physician assistants and nurse practitioners from across the country to review complex atopic dermatitis, psoriasis, chronic hand eczema, and hidradenitis suppurativa patient cases.
Panelists discuss how twice-daily dosing represents the biggest adherence challenge and how simplifying regimens from multiple location-specific medications to single versatile treatments significantly improves adherence, with once-daily formulations being much more manageable for busy families.

Panelists discuss how roflumilast demonstrated 25% Investigator’s Global Assessment (IGA) success at 4 weeks in the Integument PED trial with impressive long-term results showing 73% Eczema Area and Severity Index (EASI-75) achievement at 56 weeks, while highlighting its excellent tolerability profile with no black box warning, no folliculitis, and minimal stinging compared with previous phosphodiesterase-4 inhibitors.

Findings suggest immune dysregulation and chronic inflammation in atopic dermatitis may contribute to increased skin cancer susceptibility.

Skin pain in atopic dermatitis significantly impacts quality of life, necessitating its recognition and treatment as a vital symptom for better patient outcomes.

Castle Biosciences’ new test uses molecular signatures from lesional skin to guide systemic therapy decisions in moderate to severe AD.

Discover the latest advancements in atopic dermatitis treatment and patient advocacy during Eczema Awareness Month, shaping future care and quality of life.

Panelists discuss how tapinarof showed strong efficacy in the Adoring trials, with 45% to 46% Investigator’s Global Assessment (IGA) success and 55% to 60% Eczema Area and Severity Index (EASI-75) achievement at 8 weeks. However, they question real-world adherence to daily application over such extended periods and note folliculitis as a notable adverse effect occurring in over 5% of patients.

Panelists discuss how ruxolitinib cream demonstrated impressive efficacy in the TRU83 trial, with over 50% of patients achieving near-complete clearance at 8 weeks using the 1.5% formulation, while acknowledging that the Janus kinase (JAK) inhibitor’s broad mechanism makes its effectiveness unsurprising despite concerns about the boxed warning.

Christopher Bunick, MD, PhD, urged clinicians to move beyond therapeutic inertia and transition patients to advanced systemic therapies.

Arcutis has expanded roflumilast cream in the US, a steroid-free treatment for mild to moderate AD in young children.

Incyte reveals promising TRuE-AD4 trial results for ruxolitinib cream, showcasing its effectiveness and safety for adults with moderate atopic dermatitis.

At Fall Clinical 2025, Mona Shahriari, MD, presented highlights on the role of IL-13 inhibitors, such as dupilumab, tralokinumab, and lebrikizumab, in managing atopic dermatitis across severities.
James Del Rosso, DO; April Armstrong, MD, MPH; Mark Nestor, MD, PhD; Mark Lebwohl, MD; Linda Stein Gold, MD; and Darrell Rigel, MD, MS, presented some of the biggest updates in dermatology to close out the year.

At a recent Dermatology Times Case-Based Roundtable event, John Browning, MD, led a discussion on evolving strategies for atopic dermatitis management.
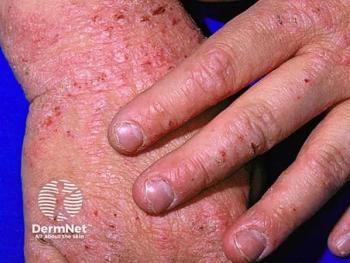
The findings reinforce dupilumab as a safe, long-term cornerstone therapy for pediatric AD, with emerging flexibility in treatment scheduling.
Experts discuss the OX40 pathway's potential to transform atopic dermatitis treatment, addressing unmet needs and promising new therapies for better patient outcomes.

In the ARCADIA LTE, nemolizumab maintained significant and progressive improvements in skin and itch outcomes for up to 104 weeks.

Mobile app SkinTracker enhances atopic dermatitis care through remote assessments, proving effective and convenient for patients while saving costs.
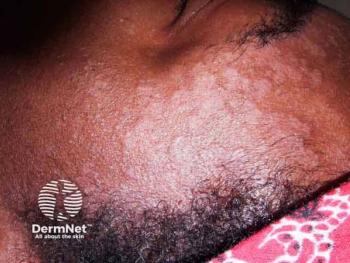
A novel 4% glycyrrhetinic acid scalp gel shows rapid relief for mild to moderate seborrheic dermatitis, enhancing treatment options with herbal ingredients.

During a Case-Based Roundtable event, Naiem Issa, MD, PhD, guided colleagues through 3 complex atopic dermatitis cases, highlighting how modern topicals can deliver rapid and durable control across age groups.

Panelists discuss how OX40-pathway therapies may serve as first-line treatments after topicals due to their promising efficacy and safety profiles, though questions remain about optimal patient selection, long-term safety, and predictive biomarkers.

Panelists discuss how multiple OX40 pathway–targeted drugs are in development, including rocatinlimab (anti-OX40, cell depleting) and amlitelimab (anti-OX40L, nondepleting), both showing promising phase 2/3 results with potential for sustained responses.

Radiation dermatitis significantly affects breast cancer patients, impacting quality of life and treatment. Discover effective prevention and management strategies.
The FDA has approved roflumilast 0.05% cream for atopic dermatitis in children aged 2 years and older, extending its use to a critical pediatric population.