
Massimo Gadina, PhD, underscored the need for genetics-guided patient selection to improve treatment specificity in inflammatory skin diseases.
Maddi Hebebrand is an associate editor of Dermatology Times and joined the MJH Life Sciences team in May 2024. She attended Baldwin Wallace University, studying Media Production and Film, and received her Masters in Digital Media from Ohio University. When she's not writing, Maddi loves to read, attend concerts and spend time with her family.

Massimo Gadina, PhD, underscored the need for genetics-guided patient selection to improve treatment specificity in inflammatory skin diseases.
Michael Lewitt, MD, emphasizes adherence to twice-daily application as the primary determinant of clinical success.

Dupilumab clears AD clinically and histologically, but deep immune profiling shows disease-memory T cells persist in the skin.

New research reveals ritlecitinib's potential to reverse scarring alopecias by targeting inflammation, offering hope for hair regrowth in autoimmune disorders.
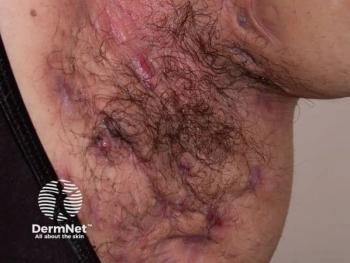
Discover the latest insights on hidradenitis suppurativa treatment, emerging therapies, and the importance of compassionate care for patients.

Benjamin Ungar, MD, shares groundbreaking insights on seborrheic dermatitis and emerging treatments in dermatology at ISDS 2025.
Using multiple treatment modalities often achieves superior repigmentation compared with monotherapy, according to Michael Lewitt, MD.

Experts discuss hair loss challenges and treatments for skin of color, highlighting the importance of early intervention and accurate diagnosis.

Neuroimmune modulation, including vagus nerve stimulation, is highlighted as a promising strategy for patients unresponsive to biologics.
Kilian Eyerich, MD, PhD, argues that classifying skin diseases by immune response patterns offers clearer therapeutic guidance than traditional morphology.

Michael Rosenblum’s team identified a previously underappreciated fibroblast subset that actively regulates inflammation, fibrosis, and microbial containment in the skin.
Therapeutic modulation of mitochondria offers a pathway to control multiple cytokine pathways simultaneously.

Chronic itching can reflect systemic disease, highlighting the importance of comprehensive assessment.
Robert Bissonnette, MD, presented tape-strip transcriptomics detecting meaningful steroid responses after just 1 day of therapy.

Kim explores the significance of inflammation in dermatology, revealing its connections to various diseases and the importance of focused research.
The study supports the hypothesis that targeting oxidative stress could complement phototherapy in restoring pigmentation in vitiligo.

Marc Serota, MD, notes that areas lacking hair follicles respond more slowly to therapy and benefit from long-term, low-risk use of topical ruxolitinib.

Nemolizumab shows significant efficacy in reducing pruritus and improving skin clearance in patients with prurigo nodularis, offering new therapeutic hope.
Experts discuss essential skin care strategies for sensitive skin, emphasizing evidence-based cleansing and moisturizing to restore the compromised skin barrier.

Lindsay Ackerman, MD, FAAD, shares practical insights on managing vitiligo with topical ruxolitinib, addressing patient expectations, safety, and evolving research gaps.

The topline data support advancing INF904 into larger, controlled trials as a potential convenient alternative to injectable biologics.

Experts discuss icotrokinra's potential to revolutionize psoriasis treatment with oral therapies, emphasizing efficacy, safety, and patient-centered care at EADV 2025.

Findings suggest immune dysregulation and chronic inflammation in atopic dermatitis may contribute to increased skin cancer susceptibility.

An AI-powered handheld spectroscopy device is showing promise in improving melanoma detection accuracy among primary care physicians, addressing critical gaps in early diagnosis and timely referral.

Clinical trials DELTA 1, 2, 3, and DELTA FORCE demonstrated significant improvements in CHE severity and patient-reported outcomes with delgocitinib.
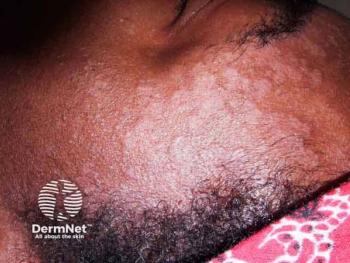
A phase 2 extension trial found roflumilast foam safe and effective for up to 52 weeks in patients with moderate to severe seborrheic dermatitis.

Lindsay Ackerman, MD, explains the importance of transparency when prescribing medications with boxed warnings.

Castle Biosciences’ new test uses molecular signatures from lesional skin to guide systemic therapy decisions in moderate to severe AD.

From acne to psoriasis, nutrition influences disease severity, treatment response, and patient quality of life.

Phio Pharmaceuticals reveals promising results for PH-762, a novel siRNA therapy targeting skin cancer, showcasing effective tumor clearance and safety.