
Gilead announces results from their phase 3 trial investigating different dosing durations for the investigational antiviral drug remdesivir.

Gilead announces results from their phase 3 trial investigating different dosing durations for the investigational antiviral drug remdesivir.

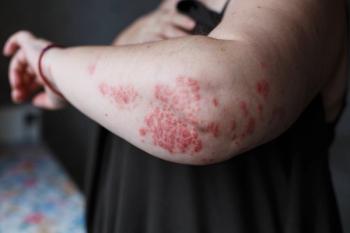
A new symptom is popping up in COVID-19 patients. The virus can reportedly affect not only the lungs, but other internal organs as well as the senses – and now dermatologists are observing skin reactions in patients with the disease.

George Martin, M.D., dermatologist and program director for the Maui Derm meetings, has been working with the Maui Derm faculty, immunologists and members of industry to share emerging data regarding the novel coronavirus with the dermatology community. Listen to his insights in this video.

In combatting the pandemic, dermatologists must take critical steps to adapt operations while still providing essential patient care and preventing exposure in o ffice settings, experts say.

Jules Lipoff, M.D., dermatologist and assistant professor of clinical dermatology, Penn Medicine, discusses telemedicine in the era of COVID-19.

Governments and the scienti fic community are racing to identify new and existing drugs that might help flatten the curve and save lives. Read from experts as they discuss the latest news and research surrounding possible treatments for COVID-19.

The American Medical Association announces new resources for private practice physicians during the COVID-19 pandemic.

Experts sent a letter to the White House Coronavirus Task Force March 24, outlining concerns about a growing shortage of medications required for managing patients with lupus and rheumatoid arthritis after President Donald Trump said in a March 19 press conference that the anti-malaria drug hydroxychloroquine could treat COVID-19.
As the number of patients infected with COVID-19 continues to rise, several experts are relaying data they say may save lives and change the course of this pandemic. According to Drs. George Martin, Ted Rosen, Sheila Fallon-Friedlander, Albert Yan, and James Treat, recent data provides evidence of the use of HCQ, its dosing, as well as epidemiology insights that will serve all healthcare practitioners in the fight against the novel coronavirus, COVID-19.
The Journal of Drugs in Dermatology is making additional resources available to medical professionals who wish to continue their education in light of medical conference cancelations due to COVID-19.

As the national pandemic continues to escalate, the American Academy of Dermatology is actively compiling resources related to the novel coronavirus to support its members and their practices.

The American Academy of Dermatology (AAD) has issued interim recommendations for practitioners to balance the risk of immunosuppression with the risk of disease flare amid COVID-19 pandemic.
The Dermatology Innovation Forum has announced its cancellation amid global spread of the coronavirus.

The American Academy of Dermatology Board of Directors has decided to cancel the 2020 Annual Meeting in Denver due to coronavirus concerns.

It’s known that combination therapies can be used to create ideal treatment outcomes, but according to Dr. Suneel Chilukuri, results may also last longer than originally thought.


Millennials think of aesthetic treatments no differently than other beauty appointments, according to Dr. Ava Shamban. These are their top facial concerns.

The FDA announced it has accepted a priority review of dupilumab for the treatment of moderate-to-severe AD in pediatric patients 6 to 11 years of age. The drug is already approved for treatment of AD in adolescents and adults.

In a skit depicting Carnac the Magnificent, Drs. Rosen and Bhatia presented on 2020's new drugs and technologies at the current Maui Derm for Dermatologists, all with a magical twist.
Investigative cryolipolysis technique holds promise as a new strategy for selective, nonsurgical fat reduction.

In his presentation at the current Maui Derm 2020, Dr. Curtis Cole tackled the controversy of how SPF labeling can differ significantly from the actual protection.

An analysis by Vanguard Communications suggests more weight is attached to physician patient reviews compared to those of hotels and restaurants.

South Florida practitioner Dr. Susan Weinkle received the JDD Outstanding Educator & Mentor in Dermatology at the ODAC held Jan.17-20 in Orlando, Fla. The award recognizes continuing contributions to the dermatology industry through education.

Verrica released positive data from its post-hoc pooled analysis of pivotal phase 3 trials investigating the safety and efficacy of VP-102 for the treatment of molluscum contagiosum.

Medical device company, Soliton will present results from their 12-week study looking at the treatment of fibrotic scars using their RAP device at Maui Derm Jan. 25-29.

Hans Hofland, Ph.D, and Dr. Steve Xu shine a spotlight on Dermira and its novel topical medicine indicated for primary axillary hyperhidrosis in patients 9 years of age and older.

The distribution agreement between Revance and Swiss HA-based dermal filler and cosmeceutical manufacturing company, TEOXANE SA, may position Revance as a leader in the U.S. facial injectable market.

A variety of topical CBD products are becoming increasingly available to patients, but do they actually work? These experts believe more research is needed to create CBD-containing dermatology products that can effectively treat skin disorders.

Prebiotics, probiotics, postbiotics and peptides - according to Dr. Manjula Jegasothy, all eyes should be on these.
Results from an EU phase 3 clinical trial of a combination of betamethasone dipropionate and calcipotriene cream showed improved patient convenience, quality of life and efficacy compared to a betamethasone dipropionate and calcipotriene gel for patients with plaque psoriasis.