
In this week’s edition, we feature stories about black-owned skincare lines, polyglutamic acid, hair antioxidants, a skincare company making affordable custom topical prescriptions, plus many more.

In this week’s edition, we feature stories about black-owned skincare lines, polyglutamic acid, hair antioxidants, a skincare company making affordable custom topical prescriptions, plus many more.

ICYMI, we've curated a list of all the stories we've published throughout the past week. Check out this article for more Dermatology Times content.

LEO Pharma will soon begin marketing its monoclonal antibody tralokinumab for atopic dermatitis with the announcement that the EMA has accepted the marketing authorization application for the drug.
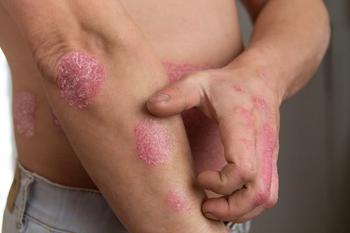
Dermavant announces the enrollment completion for their long-term safety study investigating tapinarof in adults with plaque psoriasis as a part of the PSOARING 1 and PSOARING 2 phase 3 program.

AAD announces two physicians are recipients of the "Patient Care Hero" award for their work in implementing a teledermatology program in the U.S. Army program two decades ago.

In part two of our interview with Iltefat H. Hamzavi, M.D., he delves into the data from a recent article investigating the use ultraviolet germicidal irradiation (UVGI) as a method to disinfect N95 respirators for reuse during the COVID-19 pandemic.

In this webinar, moderated by Daniel Siegel, M.D., M.A., FAAD, FACMS, experts discuss guidance on the use of teledermatology and ways to successfully implement new models of care during the COVID-19 pandemic.

Eighteen residents and fellows in the throes of their dermatology training volunteered at the frontlines of overwhelmed Bronx-based hospitals during the height of the COVID-19 pandemic, gaining a crash course in crisis medicine and infectious diseases.

In part one of our video interview with Iltefat H. Hamzavi, M.D., he discusses how ultraviolet germicidal irradiation is used in dermatology and how it can be used to disinfect N95 respirators to facilitate reuse during the COVID-19 pandemic.

ForBabs introduces their X-Static hairbrush that keeps fly-aways and frizz at bay.

In this week’s edition, we highlight stories about ways to support people of color in the beauty industry aside from purchasing products, the safety of LED light therapy, dermaplaning at home, why your skin might be acting up right now, plus more.

Efforts are underway to identify new therapeutic methods that can offer improved outcomes for patients with hidradenitis suppurativa.

Efforts are underway to identify new therapeutic methods that can offer improved outcomes for patients with hidradenitis suppurativa.

In case you missed it, this week we featured stories involving blastic plasmacytoid dendritic cell neoplasm (BPDCN), development of a biosimilar to BOTOX, a new nonmelanoma treatment device, the latest phase 3 results for abrocitinib in atopic dermatitis, results from a long-term dabrafenib and trametinib study in stage 3 BRAF-mutated melanoma, plus many more.

In honor of June being Acne Awareness Month, Thayers has launched a new line of acne-tailored skincare products.

The American Society for Laser Medicine and Surgery announces a new president and officers will be joining their Board of Directors for 2020-21.

Dermatology patients are hungry for information that will ensure their safety during the COVID-19 pandemic, notes Vivian Shi, M.D. In this video, she discusses how to address patients' varying concerns in this uncertain time.

In the final installment of our interview with Amy Paller, M.D., she explains the similarities and differences between Kawasaki disease and the novel condition, multisystem inflammatory syndrome in children (MIS-C).

Part three of our interview with Amy Paller, M.D., focuses on the emergence of a multisystem inflammatory syndrome in children (MIS-C), what we know about this novel condition and its relationship to COVID-19.

DermYoung and Eden MedSpa announce the launch of their “Together We Are Love, Together We Will Heal,” campaign which aims to provide NYC first responders with facials and take-home skincare kits for those experiencing skin damage and irritation due to PPE usage.

Bluestone Sunshields is helping fill the gaps of PPE available to those working on the frontlines of the COVID-19 pandemic by converting their UV ray-blocking visors into clear face shields.

In this week's edition we take a look at the world of injectables, the effects diabetes can have on the skin and how to correctly administer an at-home chemical peel.

In part two of our interview with Amy Paller, M.D., she describes how she is staying informed about the latest COVID-19 research as it relates to the field, and shares some resources other dermatologists may find helpful during this crisis.


DMK Skin Care announces the donation of their new sanitizer spray for all licensed clinics. The product is optimized to sanitize clothing, surfaces and skin.

Amy Paller, M.D., discusses the pernio-like lesions associated with possible COVID-19 infection in younger individuals.
The U.S. FDA has granted an emergency use authorization to Everlywell Inc., to conduct and collect at-home testing for COVID-19.

The American Academy of Dermatology (AAD) announces the debut of their AAD VMX 2020, a virtual meeting being held June 12-14 in lieu of the annual AAD conference that was set to take place in March in Denver but cancelled due to COVID-19 concerns.

A new way to avoid contact with contaminated surfaces is now available through 5th Axis, a San Diego, Calif.-based machinery manufacturer. The company has created the StepHandle, a door handle that can be operated with a foot.

Ortho Dermatologics announces the U.S. FDA supplemental New Drug Application approval for efinaconazole 10% (JUBLIA) for the treatment of onychomycosis, a fungal toenail infection, in patients 6 years and older.