
A glimpse at what to expect from Dermatology Times this week.

A glimpse at what to expect from Dermatology Times this week.

ICYMI, some of this week’s featured content includes articles on the multiple modalities to revise scars, the future of dermatology, phase 3 results of tapinarof cream for plaque psoriasis, guidelines for the COVID-19 vaccine and dermal fillers, plus more.

ASDSA has issued a patient safety alert after videos surface online of children/teens self-injecting HA via “hyaluron pens”.

Joel Cohen, MD, gives a synopsis of a panel discussion he was recently a part of at the 2021 ODAC Dermatology, Aesthetic and Surgical Conference focusing on the postoperative treatment of scars using a variety of methods including lasers, filler and other modalities.

In this episode, Gary Salman, CEO of Black Talon Security, details how to best protect your practice from data breaches and cybercriminals, as well as the risks of not implementing cybersecurity measures into your practice and what to do if a data or email breach occurs.

Three experts chart game-changing innovations that will shift the boundaries of dermatology.

Treatment regimens for pediatric acne should consider skin type, ethnicity, disease severity and personal preferences, according to one expert.

This week’s edition of The Mainstream Patient features stories on hidradenitis suppurativa, the rise of contact dermatitis, sebaceous filaments, coconut oil for the face, plus more.

A glimpse at what to expect from Dermatology Times this week.

In case you missed it, some of this week’s featured content includes the latest FDA approvals, newly published phase 3 data for tirbanibulin, oxytocin and its effect on skin aging, plus more.

Nicole Hayre, MD, FAAD, discusses her recent research related to oxytocin's effect on skin aging and how increased levels of oxytocin in the body can make the skin appear younger.

Topical probiotics show promise in treating various skin diseases including acne, but more research is needed.

Spending time around animals as an infant has been suggested as a way to reduce the incidence of atopic dermatitis. An investigation offers some insight.

The FDA has approved cemiplimab-rwlc as the first immunotherapy for use in patients with advanced basal cell carcinoma that has previously been treated with a hedgehog pathway inhibitor (HHI) or for whom a HHI is not appropriate.
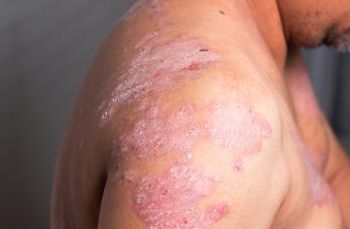
According to one expert, recently completed phase 3 trials of novel drugs have shown impressive results, with 2021 expected to be an exciting year that will bring further advances in treatment options for patients with moderate-to-severe plaque psoriasis.

This week’s edition of The Mainstream Patient features stories on tretinoin, dermal fillers, dermarolling, niacinamide, and more.

A glimpse at what to expect from Dermatology Times this week.

In case you missed it, some of this week’s featured content includes a new podcast episode on the popularity of TikTok for dermatologists, FDA approvals for Soliton’s RAP device for cellulite and Galderma’s Restylane Defyne for chin augmentation, enrollment commencement for a food supplement and skin inflammation study, plus more.

In this month's Cosmetic Conundrums column, Dermatology Times Chief Medical Editor Zoe Diana Draelos, MD, shares her tips for using cosmetics under face masks.

Galderma announces the FDA-approval of HA dermal filler, Restylane Defyne for use in chin augmentation.

A proprietary food supplement designed to balance the gut microbiome and target the over production of skin cells will be examined in an upcoming consumer study that has recently announced participant enrollment.

Soliton announced the FDA has cleared its Rapid Acoustic Pulse (RAP) device for short-term improvement in appearance of cellulite.

This week’s edition of The Mainstream Patient features stories on sunscreen expiration, the link between dairy and acne, the skin barrier, how to manage retinization, plus more.

A glimpse at what to expect from Dermatology Times this week.

In case you missed it, some of this week’s featured content includes a new podcast episode, a video series on the COVID-19 vaccine and dermal fillers, topical PRP, expansion of the CoolSculpting portfolio, combination therapies as the future of rosacea treatment, plus more.

Experts are recommending combination therapies as a way to treat rosacea, according to a presentation at ODAC Dermatology, Aesthetic and Surgical Conference 2021 Pre-Conference Sneak Peek Inflammatory Diseases Virtual Symposium.

In the final part of our interview with Whitney Bowe, MD, she reveals her tips and tricks for both preventing and minimizing any adverse reactions in her filler patients who might have also received the COVID-19 vaccine.
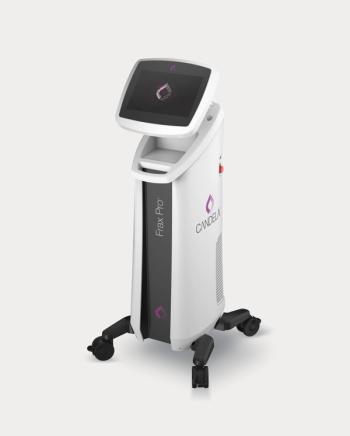
Candela announces the launch of its Frax Pro system, featuring dual-depth skin resurfacing with both novel Frax 1940 and Frax 1550 applicators.
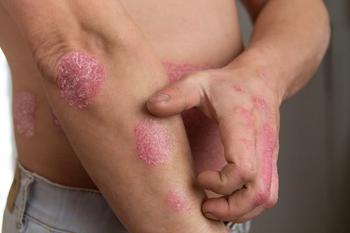
Safety profiles remain consistent longer-term but cost and accessibility are major challenges for biologics aimed at treating psoriasis.

In part 4 of our interview with Whitney Bowe, MD, she dives into the multiple methods physicians can use to treat adverse reactions from the COVID-19 vaccine in their dermal filler patients.