
Discover essential dermatology insights from experts at the Fall Clinical Conference 2025, featuring innovative treatment tips and patient care strategies.

Discover essential dermatology insights from experts at the Fall Clinical Conference 2025, featuring innovative treatment tips and patient care strategies.

Emerging skin treatments present unique clinical challenges that must be anticipated by clinicians.

New data presented at Fall Clinical 2025 reveal that patients in socioeconomically disadvantaged areas are significantly less likely to initiate biologic or small molecule treatments.

Recent FDA approvals for topical roflumilast and ruxolitinib down to age 2 mark major milestones in managing pediatric AD, according to Peter Lio, MD.

Catch up on coverage from the second day of the 2025 Fall Clinical Dermatology Conference held in Las Vegas, Nevada.
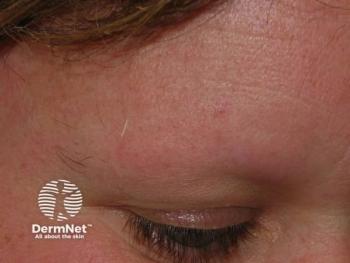
At 52 weeks, the data presented at Fall Clinical showed that 64.8% and 63.3% of adolescents on baricitinib 4 mg achieved significant eyebrow and eyelash regrowth, respectively.

At Fall Clinical 2025, Harrison Nguyen, MD, MBA, MPH, outlined how gene expression profile testing complements traditional staging systems by helping clinicians better identify melanoma and cSCC patients at the highest risk for recurrence.

Del Rosso recounts the meeting’s evolution and its consistent mission to bridge science and practice.

New data from Johnson & Johnson's ICONIC-TOTAL study reveals icotrokinra's promising long-term efficacy and safety for treating challenging plaque psoriasis.

The AHEAD guidelines promote a new treatment standard for atopic dermatitis, emphasizing near-complete skin clearance and itch relief.

Data from Fall Clinical 2025 detailed the results from the phase 2 STRIDE and ongoing open-label extension studies demonstrating the efficacy and safety of envudeucitinib, a selective TYK2 inhibitor.

AVAVA receives FDA clearance for wrinkle treatment, showcasing significant results and high patient satisfaction with its innovative Focal Point Technology.

Catch up on coverage from the first day of the 2025 Fall Clinical Dermatology Conference held in Las Vegas, Nevada.

A new IPC-endorsed framework simplifies psoriasis severity into “topical” versus “systemic” categories to guide treatment more effectively, according to James Song, MD.
James Del Rosso, DO; April Armstrong, MD, MPH; Mark Nestor, MD, PhD; Mark Lebwohl, MD; Linda Stein Gold, MD; and Darrell Rigel, MD, MS, presented some of the biggest updates in dermatology to close out the year.
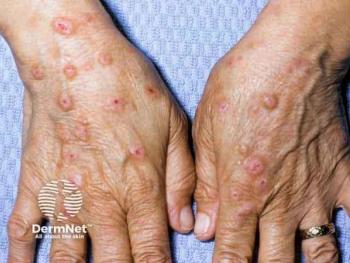
PN is increasingly recognized as a common and severe inflammatory skin disease disproportionately affecting patients with darker skin tones.

Click through this slideshow to see what faces familiar to Dermatology Times will be on the speaker stand at Fall Clinical this week.
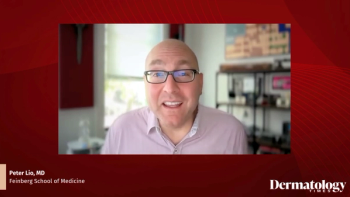
Peter Lio, MD, describes Fall Clinical as an energizing, high-level dermatology meeting focused on practical takeaways and collaboration.
Biologics, JAK inhibitors, and evolving treatment paradigms headline the Fall Clinical 2025 agenda.

The 25th annual meeting begins today and runs through October 26.

Explore real-world strategies with Leigh Ann Pansch, MSN, FNP-BC, DCNP, for managing psoriasis, emphasizing patient-centered care, and implementing individualized biologic treatment approaches.

Surface keratin 1 has emerged as a promising target in triple-negative breast cancer, bridging dermatology and oncology for innovative treatments.

Fall Clinical 2025 continues its tradition of blending cutting-edge science with practical insights for the modern dermatologist.
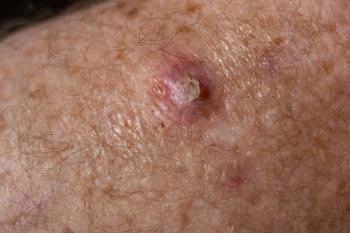
Medicus Pharma has initiated a phase 2 trial in the United Arab Emirates (UAE), exploring noninvasive treatment for basal cell carcinoma (BCC) using innovative microneedle technology.

Jamie Restivo, MPAS, PA-C, shares her key takeaways from the first day of the recent Pennsylvania Dermatology Physician Assistants (PDPA) Keystone Dermatology Conference in Philadelphia, Pennsylvania.

Discover non-clinical career paths for PAs and NPs at the 2025 PDPA Keystone Conference, featuring insights from Shanna Miranti, MPAS, PA-C, and Lauren Miller, PA-C.

CO₂ laser treatment significantly enhances quality of life for hidradenitis suppurativa patients, showing promising outcomes in a large prospective study.

At a recent Dermatology Times Case-Based Roundtable event, John Browning, MD, led a discussion on evolving strategies for atopic dermatitis management.

New evidence supports biologic agents as precise, better-tolerated alternatives to systemic immunosuppressants for life-threatening dermatologic emergencies.

This October, discover how dermatology and oncology collaborate to enhance breast cancer care and support patients through innovative therapies and teamwork.