
Explore recent research that shed light on potential associations and manifestations post-COVID-19 infection or vaccination.

Exploring the Relationship Between Oligodontia and Hair Abnormalities: A Study Overview

Explore recent research that shed light on potential associations and manifestations post-COVID-19 infection or vaccination.

Joseph Zabinski, PhD, MEM, of OM1, shares insights at AAD into how artificial intelligence can mature and be a positive tool for disease detection.

The congenital ichthyosis session the 2024 AAD Annual Meeting covered real-world patient cases and treatments on the horizon.
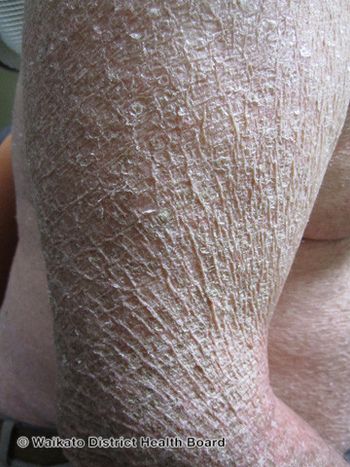
Christopher Bunick, MD, PhD, provides tips for the general dermatologist to treat ichthyosis and shares data on TMB-001 in development for ichthyosis.

Click here to answer our poll on rare dermatologic diseases.

Today is Rare Disease Day. Here's a look back at news related to rare skin conditions over the past year.

This Rare Disease Day, Joseph Zabinski, PhD, MEM, of OM1, discusses the role of artificial intelligence in helping to detect rare dermatologic diseases.

This Rare Disease Day, Stefan Weiss, MD, MBA, FAAD, of OM1, shares his expertise in AI’s capabilities in dermatology.
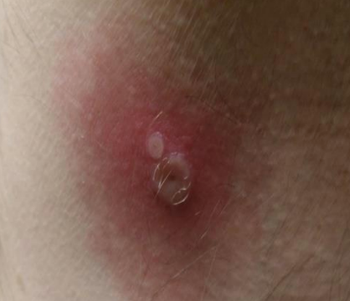
Officials recently reported the first known fatality from the virus. Here's what health professionals say to watch for.

From the February cover: Joseph Zabinski, PhD, MEM, and Stefan Weiss, MD, MBA, FAAD, share their expertise into AI’s capabilities in dermatology and specifically, rare diseases such as generalized pustular psoriasis.

Krystal Biotech’s B-VEC was administered as an eyedrop for a patient with dystrophic epidermolysis bullosa with cicatrizing conjunctivitis.

Factors such as nutritional compromise, low levels of vitamin D, and more, negatively affect bone metabolism in this patient population.

J3401 went into effect on January 1, 2024.
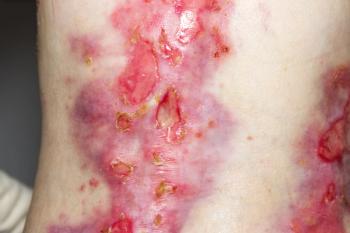
Chiesi Global Rare Diseases recently announced the approval of the topical treatment.
With greater understanding of this rare pathology and its signs and symptoms, review authors believe this understanding could prompt early and necessary intervention.

The FDA’s PDUFA target date is May 25, 2024.

There are currently no FDA-approved therapies for the rare, genetic disease.

The PC Project provides patients with access to the International PC Research Registry for clinical help with their rare and painful skin condition.

Kyverna Therapeutics can now initiate its phase 1/2 open-label, multicenter study of KYV-101.

A BLA is expected to be submitted by Q3 of 2024.

Timber Pharmaceuticals has been developing TMB-001 for the treatment of congenital ichthyosis.
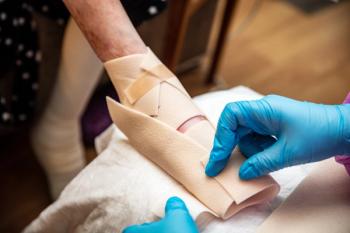
The company is set to meet with the FDA in August to discuss the epidermolysis bullosa drug’s anticipated BLA.

Patients with the rare autosomal recessive genodermatosis are prone to extreme photosensitivity and changes in skin pigmentation.

Researchers conducted a review of treatments for the rare disease in the pediatric population.

New pharmacokinetic phase 3 data will be presented at the 2023 Society for Pediatric Dermatology meeting next week.