New pathways of care are needed to improve health care spending.

New pathways of care are needed to improve health care spending.
At the 2022 Fall Clinical Dermatology Conference, Bruce E. Strober, MD, PhD, reviewed the best treatment options for patients with psoriasis and psoriatic arthritis.
Two expert dermatologists discuss the relationship between PsA and PsO in this DermView series recap.

Janssen Pharmaceutical Companies of Johnson & Johnson announced the approval of ustekinumab as a new treatment option for patients aged 6 years or older with active psoriatic arthritis.
Arthur Kavanaugh, MD, a professor of medicine and director of the Center for Innovative Therapy (CIT) at UC San Diego in California, gives his thoughts on the treatments for psoriatic arthritis, the comorbidities that have to be considered, and more.
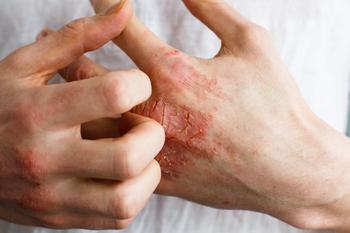
Arthur Kavanaugh, MD, a professor of medicine and director of the Center for Innovative Therapy (CIT) at UC San Diego in California, gives his thoughts on the future of psoriatic arthritis.
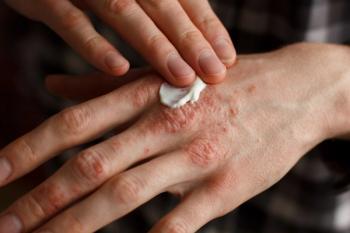
At the SDPA 2022 Annual Summer Dermatology Conference, a session on both common and rare dermatological disorders.

Tralokinumab-ldrm (Adbry; LEO Pharma) showed improvement in itch, sleep, anxiety, depression, and overall quality of life data for adolescent AD patients in newly released study data.

AbbVie announced the FDA approval to their IL-23 inhibitor risankizumab-rzaa (Skyrizi) for treatment of active psoriatic arthritis (PsA) in adult patients based on the results of the KEEPsAKE-1 and KEEPsAKE-2 trials.

Secukinumab is the only biologic treatment in the US that’s approved by the FDA for pediatric patients with enthesitis-related arthritis (ERA) and psoriatic arthritis (PsA).

A study in the Journal of the American Academy of Dermatology examined the incidence of psoriasis according to body mass index.

A recent study investigated apremilast use for psoriasis during COVID-19 infection.

The FDA approves upadacitinib (Rinvoq; AbbVie) for the treatment of adult patients with active psoriatic arthritis.

The study examines the impact of antirheumatic treatment on pregnancy outcomes in women with psoriatic arthritis.
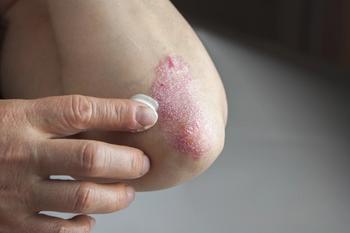
Mark G. Lebwohl, MD, presents on what dermatologists need to know about the newest psoriasis studies during the Fall Clinical Dermatology Conference.

Mark Lebwohl, MD, takes a deep dive in the recent delay in approval for multiple JAK inhibitor drugs for treatment of inflammatory skin conditions such as psoriasis and atopic dermatitis. He also discusses the impact of the delay, as well as the benefits of JAK inhibitors for inflammatory skin diseases, and an outlook for the future of this drug class.

The FDA is now requiring black box warning for certain JAK inhibitors used to treat arthritis. This news comes amid delay in FDA approval for multiple JAK inhibitors for the treatment of inflammatory skin conditions.

Mark Lebwohl, MD, explains the recent delay in approval of JAK inhibitors by the FDA, how it is affecting the inflammatory skin disease community, and if physicians should be concerned.

As COVID-19 eases, the FDA is breaking through its backlog and clinical trials are moving forward, opening the way for a new wave of treatments.
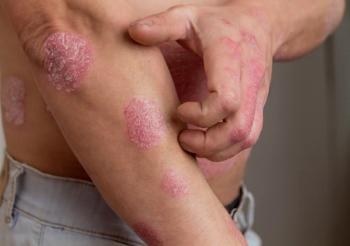
Amgen and the International Federation of Psoriasis Associations have announced their efforts to combat the challenges of psoriasis.

Patients with anxiety/depression and psoriatic arthritis (PsA) are much less likely to achieve long-term remission. Mental health comorbidities need to be a more important part of PsA treatment.

The FDA announces it did not meet the June 25 PDUFA action date on the sNDA for active psoriatic arthritis treatment, upadacitinib.
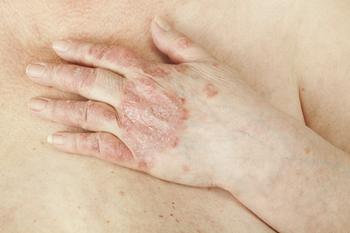
COSMOS phase 3b study data demonstrated the effectiveness of guselkumab in patients diagnosed with psoriatic arthritis.

Biologic therapy has proven very useful in treating an increasing number of dermatologic diseases and conditions. However, caution is warranted for patients with chronic infectious diseases.

Novel biologics are moving into off-label indications to expand the dermatologic armamentarium for a broad range of inflammatory skin diseases.