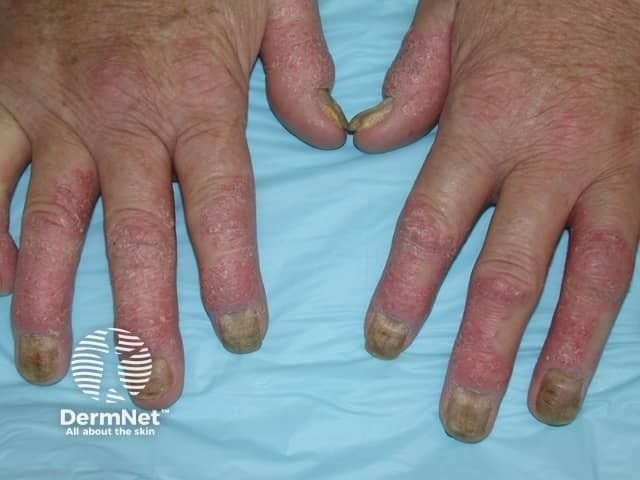
Psoriatic Arthritis
Latest News
Latest Videos

CME Content
More News

Otulfi from Formycon and Fresenius Kabi was approved simultaneously in the US and the European Union. In the US, it will launch in February 2025.

Mease, the lead investigator on the trial, spoke about comparing varying mechanisms of action, primary and secondary endpoints, and how the results of BE BOLD could affect treatment choices.

This marks the first head-to-head trial of an IL-17A/F inhibitor against an IL-23 inhibitor in psoriatic arthritis treatment.

Bimzelx was also approved for non-radiographic axial spondyloarthritis and ankylosing spondylitis.

Brenda Kong-Tunac, a patient with PsO, PsA, and skin of color, shares her experiences.

Researchers behind the phase 3 studies gave pearls into the study methods, results, and what is up next.

Pyzchiva will be commercialized by Sandoz in the United States.

Gottlieb reviews her lecture from the Society for Investigative Dermatology Annual Meeting.

The phase 3 IZAR program will evaluate the efficacy and safety of sonelokimab in patients with psoriatic arthritis for a 1 year duration.

Upadacitinib is now available as a tablet or an oral solution for patients 2 years and older with pJIA as well as PsA.

This research indicates that psychological well-being serves a key role in the management of psoriatic disease.

Last week, the FDA approved an sBLA for Sandoz’s adalimumab-adaz in various strengths, including 10 mg/0.1 mL, 20 mg/0.2 mL, and 80 mg/0.8 mL.

Adalimumab-adbm is now approved for use in various indications, including psoriasis, psoriatic arthritis, and hidradenitis suppurativa.

Last week, the US Food and Drug Administration approved biosimilar ustekinumab-aekn (Selarsdi; Alvotech and Teva) for psoriasis and PsA.

A matching-adjusted indirect comparison found that some patients with PsA are more likely to achieve long-term treatment outcomes with bimekizumab.
MoonLake’s sonelokimab is being evaluated for hidradenitis suppurativa, psoriasis, and psoriatic arthritis.

Up to one quarter of patients with PsA experience persistent joint pain. In a recent study, higher levels of fatigue, depression, and anxiety were associated with joint pain.

Acelyrin announced the positive results, of which the primary endpoint was met with high statistical significance, earlier this week.

Findings reveal an increased risk of psoriatic arthritis among female patients with severe skin psoriasis who have nail involvement and require oral systemic therapy for their condition.

New evidence identifies factors that may predict which patients with psoriasis will develop psoriatic arthritis.

While the first ustekinumab biosimilar, Wezlana, was approved in October 2023, a settlement with Johnson & Johnson (J&J) will keep it off the market until 2025, preventing competition, and causing purchasers to pay substantially more for the agent.
Depression, functional impairment, and worsened quality of life are related in patients with psoriatic disease, according to a recent study.

Investigators report 12 weeks of treatment provided a strong foundation for further evaluation of TAK-279 across the psoriatic arthritis disease spectrum.

The approved indication makes Wezlana the first approved interchangeable biosimilar to Stelara.
Apremilast is being examined for the treatment of psoriatic arthritis.