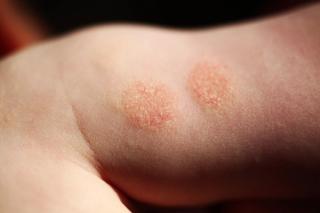
Atopic Dermatitis
Latest News
Latest Videos

CME Content
More News

Infants with eczema may require different approaches to preventing food allergies, including early introduction of peanuts to increase oral exposure to allergens before skin exposure.

Dupilumab and JAK inhibitors are leading the way in atopic dermatitis treatment, but there are numerous clinical trials of both new biologics and new small molecule drugs, and future treatment regimens likely will utilize both.

The first and only atopic dermatitis conference, Revolutionizing Atopic Dermatitis (RAD), will be hosting its third event, in a virtual format.

In a recent phase 3 trial, dupilumab (Dupixent, Sanofi and Regeneron) demonstrated positive results in itch reduction, skin clearance and quality-of-life. Indicating the biologic as a viable treatment option for severe atopic dermatitis in younger children.
A recent review reveals older adults are underrepresented in trials investigating systemic immunomodulators as a treatment for atopic dermatitis.
Adults with atopic dermatitis (AD) stay on dupilumab (Dupixent, Sanofi and Regeneron) significantly longer than on cyclosporine, according to a recent study.

Across all doses, lebrikizumab showed significant dose-dependent improvement in primary study endpoints for improvements in atopic dermatitis, according to data from phase 2b trials.

In clinical trials, most patients treated with tralokinumab maintained 75% reductions in EASI scores for one year.
Vehicles for topical atopic dermatitis treatments deliver benefits that promote barrier restoration and enhance innate immune defenses, a review suggests.

Tape strips outperformed skin biopsies by differentiating eczema from psoriasis with high accuracy, researchers report.
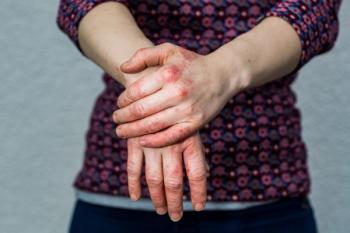
The U.S. Food and Drug Administration (FDA) has granted JAK-inhibitor delgocitinib cream (LEO Pharma) Fast Track Designation for chronic hand eczema.

Gain insight from the experts on current and future treatment for pediatric atopic dermatitis.
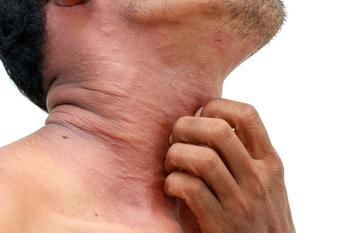
The U.S. Food and Drug administration has accepted the Biologics License Application for tralokinumab (LEO Pharma) for moderate-to-severe atopic dermatitis and is expected to make a decision in the second quarter of 2021.
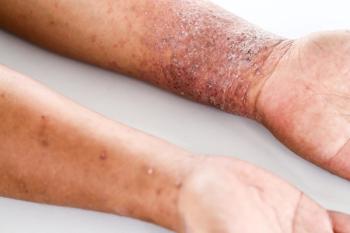
Researchers of a recent systematic review and meta-analysis examine how dupilumab compares to older systemic treatments for atopic dermatitis, including methotrexate, cyclosporine and azathioprine.

The U.S. Food and Drug Administration has approved a single-dose 300 mg pre-filled pen version of dupilumab (Dupixent, Sanofi and Regeneron) for the treatment of atopic dermatitis, asthma and chronic rhinosinusitis with nasal polyposis (CRSwNP) in U.S. patients 12 years and older.

A recent study shows no increased risk of incidence of malignancies in patients using topical tacrolimus. A black box warning on the therapy has made it challenging for physicians to prescribe, and researchers hope this evidence will lead to the FDA reconsidering that warning.
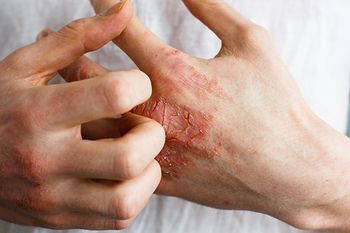
Gusacitinib, an investigational oral Janus kinase/spleen tyrosine kinase inhibitor, demonstrated statistical superiority compared with placebo for improving chronic hand eczema in a phase 2b study.
Jonathan H. Zippin, M.D., Ph.D., explains what existing drugs and investigational compounds that inhibit the JAK-STAT pathway are being investigated as treatment for atopic dermatitis, alopecia areata, vitiligo and psoriasis.

LEO Pharma will soon begin marketing its monoclonal antibody tralokinumab for atopic dermatitis with the announcement that the EMA has accepted the marketing authorization application for the drug.
Full results from the second of two trials investigating the JAK1 inhibitor abrocitinib as a monotherapy for treatment of moderate-to-severe atopic dermatitis demonstrate statistical superiority of both doses compared to placebo.

The U.S. FDA has approved dupilumab (Dupixent, Sanofi and Regeneron) for the treatment of atopic dermatitis in children 6-11 years, making it the first biologic approved for atopic dermatitis in this particular age group.

In light of the ongoing pandemic, Vivian Shi, M.D., discusses potential risks dermatologists will want to keep in mind when evaluating patients currently taking immunomodulator or immunosuppressant drugs.
Ruxolitinib met primary and secondary endpoints in the phase 3 TRueE-AD1 and TRuE-AD2 studies, underscoring the idea that the disruption of multiple cytokine networks simultaneously is a promising treatment approach.

Researchers evaluating several itch assessment tools say there is a need for a better way. Studies comparing the validity and reliability of itch measurement scales are lacking, say researchers who examined several available assessment tools.

Jonathan Silverberg, M.D., Ph.D., MPH, discusses criteria to keep in mind when evaluating a patient with possible atopic dermatitis.