Victoria Werth, MD, Discusses the Future of BDCA2 Inhibition in Cutaneous Lupus
Key Takeaways
- Hydroxychloroquine provides adequate CLE control in only ~50% of patients, and nonresponse frequently necessitates systemic immunosuppression with clinically significant toxicity and laboratory monitoring requirements.
- Escalation agents such as methotrexate and mycophenolate mofetil impose infection risk and broader safety concerns, while azathioprine may be pregnancy-compatible yet often underperforms for cutaneous disease.
Victoria Werth, MD, highlights CLE treatment gaps and how BDCA2-targeting litifilimab may quickly curb interferon-driven skin disease with less toxicity.
Victoria Werth, MD, professor of Dermatology and Medicine at the University of Pennsylvania and Chief of Dermatology at the Philadelphia VA Medical Center, met with Dermatology Times to discuss longstanding therapeutic gaps in cutaneous lupus erythematosus (CLE) and the potential role of
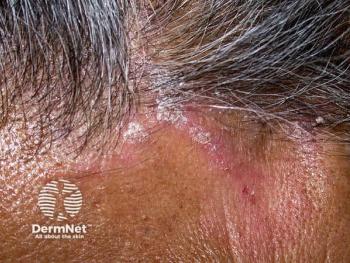
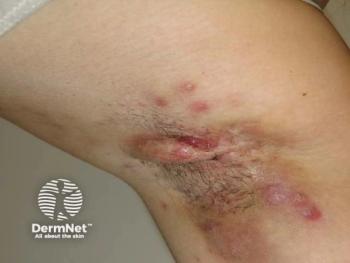
Werth emphasized that, despite decades of clinical experience, treatment advances in CLE have been limited. Many commonly used therapies, including topical and systemic corticosteroids and hydroxychloroquine, were adopted historically rather than developed through modern, rigorous clinical trial pathways. Hydroxychloroquine remains first-line, but she notes that only about half of patients achieve adequate disease control. Nonresponders frequently require escalation to systemic immunosuppressants such as methotrexate or mycophenolate mofetil, which carry clinically significant risks, including infection, potential malignancy concerns, and the need for ongoing laboratory monitoring. Other agents considered relatively safer in pregnancy, such as azathioprine, often demonstrate limited efficacy for cutaneous manifestations. As a result, many patients face a trade-off between suboptimal control and exposure to medications with substantial toxicity.
“This is a field that's really young and really needs new treatments...litifilimab may address a gap in therapy that could work potentially very quickly and be safer than most of the therapies that are currently available,” Werth said.
Litifilimab uses a targeted immunologic mechanism distinct from many existing and emerging therapies. The drug inhibits blood dendritic cell antigen 2 (BDCA2) on plasmacytoid dendritic cells (pDCs), key producers of type I interferons, which are strongly implicated in CLE pathogenesis. By reducing interferon production at the cellular source, BDCA2 inhibition modulates a central inflammatory pathway rather than broadly suppressing the immune system. Werth noted that this approach differs from therapies that primarily block cytokine receptors or downstream signaling pathways. Targeting interferon production more proximally may reduce the inflammatory drivers of skin disease while potentially avoiding some consequences of broader immunosuppression.
Werth also suggests that, mechanistically, modulation at the level of pDC activity could offer advantages in terms of disease control without triggering significant rebound phenomena if therapy is discontinued, although comparative data will be needed. Overall, BDCA2 inhibition represents a mechanistically precise strategy that aligns with evolving understanding of interferon biology in CLE and may expand the therapeutic landscape for patients with inadequately controlled disease.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.