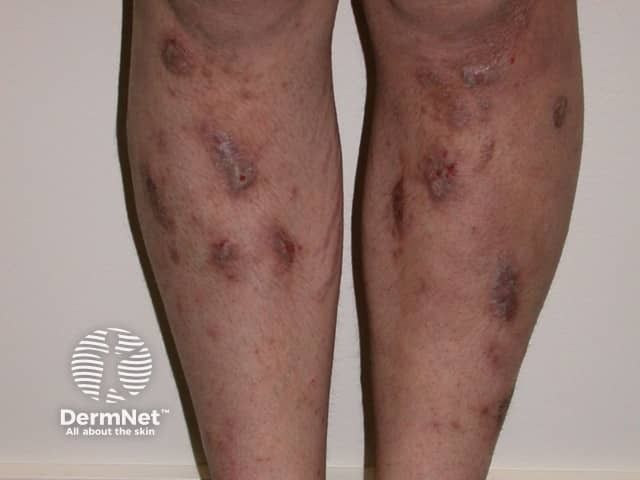
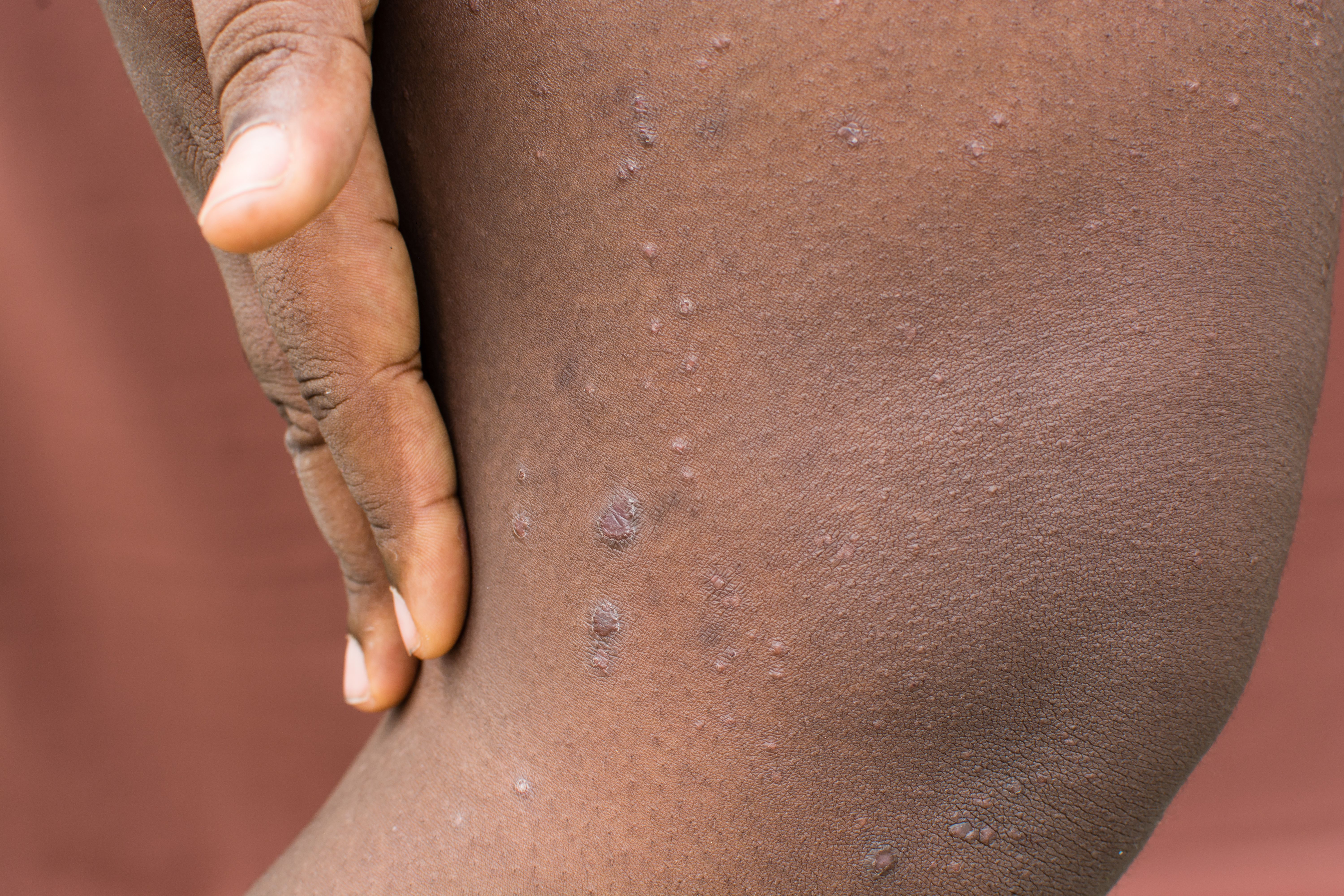
Prurigo Nodularis
Latest News
Latest Videos

CME Content
More News
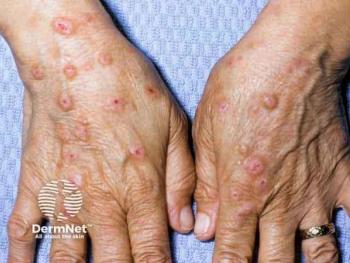
Significant reductions in itch scores and improved assessments of PN severity and quality of life were observed throughout the study.

Jason Hawkes, MD, MS, emphasized the importance of biologic treatments transforming the management of both prurigo nodularis and atopic dermatitis.

Shawn Kwatra, MD, presented the Incyte data at EADV 2024.

Catch up on the latest news on prurigo nodularis advancements in 2024.
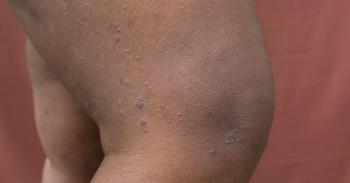
The JAK1 inhibitor provided significant relief for 2 patients with refractory PN.
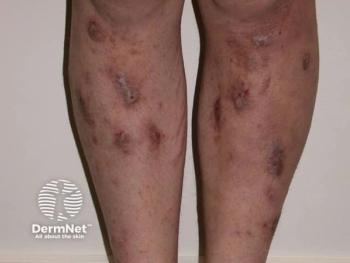
Researchers conducted a web-based survey to gain insights into current clinical practice for PN in Japan.
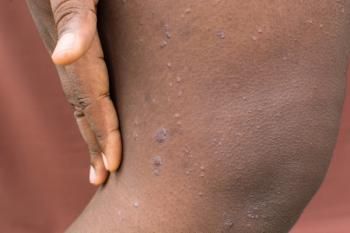
The review found that patients with PN are approximately 1.3 times more susceptible to depression and 1.9 times more likely to experience anxiety.

Galderma’s nemolizumab is the first approved monoclonal antibody specifically inhibiting the signaling of IL-31.

Scassellati Sforzolini discusses the significance of Galderma’s nemolizumab receiving 4 additional filing acceptances for prurigo nodularis and atopic dermatitis.

Povorcitinib is being evaluated for non-segmental vitiligo, hidradenitis suppurativa, prurigo nodularis, asthma, and chronic spontaneous urticaria.

Shawn Kwatra, MD, shares positive updates on phase 2 oral povorcitinib for prurigo nodularis at AAD 2024.

Shawn Kwatra, MD, presented late-breaking data at AAD 2024 on the largest prurigo nodularis and nemolizumab clinical trial, OLYMPIA.

The FDA has also granted nemolizumab Priority Review for prurigo nodularis.

A randomized, placebo-controlled trial of the selective transient receptor potential melastatin 8 agonist explored its efficacy and safety in PN.