
Galderma announces the FDA-approval of HA dermal filler, Restylane Defyne for use in chin augmentation.

Galderma announces the FDA-approval of HA dermal filler, Restylane Defyne for use in chin augmentation.

AAFPRS’s 2020 member survey examines the effect of COVID-19 on aesthetic trends, including an increase in surgical procedures, blepharoplasty, and rhinoplasty.

Galderma announces the FDA-approval of HA dermal filler, Restylane Defyne for use in chin augmentation.

Dr. Deborah L. Benzil discusses her keynote address at AACS’s upcoming second annual Women in Cosmetic Surgery event, and uplifting women in medicine.

Soliton announced the FDA has cleared its Rapid Acoustic Pulse (RAP) device for short-term improvement in appearance of cellulite.

This week, consumer magazines highlight COVID-19 vaccine effects attached to dermal filler, hyperpigmentation advice for people of color, and more.

This week in aesthetics, we highlight the launch of Galderma’s “Face for Change” program, Allergan announces the CoolSculpting Elite device and more.

In the final part of our interview with Whitney Bowe, MD, she reveals her tips and tricks for both preventing and minimizing any adverse reactions in her filler patients who might have also received the COVID-19 vaccine.
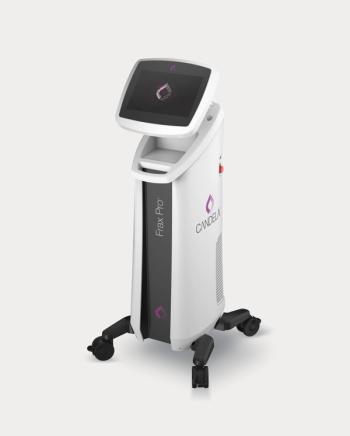
Candela announces the launch of its Frax Pro system, featuring dual-depth skin resurfacing with both novel Frax 1940 and Frax 1550 applicators.

In part 4 of our interview with Whitney Bowe, MD, she dives into the multiple methods physicians can use to treat adverse reactions from the COVID-19 vaccine in their dermal filler patients.

Recent reports have been surfacing in regard to adverse reactions in dermal fillers patients who have also received the COVID-19 vaccine. Whitney Bowe, MD, New York City, New York, discusses this phenomenon and provides some tips for preventing and treating these side effects.

Allergan announces the launch of Coolsculpting Elite, along with seven new C-shaped applicators.
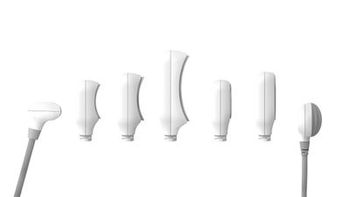
Allergan Aesthetics plans on expanding its CoolSculpting portfolio with the launch of its novel CoolSculpting Elite device that targets visible fat in nine different areas of the body.

In part 3, Whitney Bowe, MD, suggests how physicians should manage their patients' expectations when it comes to HA fillers and the COVID-19 vaccine.

Galderma announced the launch of its Face for Change program that allows abobotulinumtoxinA (Dysport) patients to give back with each treatment received to either Dress for Success or The Skin Cancer Foundation.
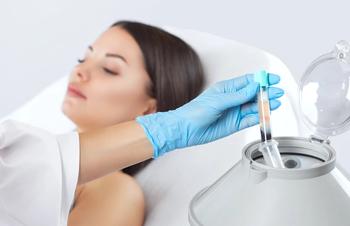
Results of a recent in vitro study show that a novel topical cosmetic vehicle successfully stabilizes and preserves platelet-rich plasma (PRP).

In the second part of our interview with Whitney Bowe, MD, she reveals the type of fillers that have been linked to adverse reactions in dermal filler patients who have received the COVID-19 vaccine.

This week, consumer magazines highlight the impact of TikTok skinfluencers, keeping a skin diary, and more.
In this expert interview, Whitney Bowe, MD, explains the rare adverse reactions the medical community has recently been seeing in dermal filler patients who received the COVID-19 vaccine.

This week in aesthetics, a study examines tofacitinib to treat alopecia areata, Dr. Michael Kluska compares Renuvion (J-Plasma) versus Bodytite, and more.

The American Society of Dermatologic Surgery releases its annual survey showing the continued popularity of minimally invasive and nonsurgical treatments.
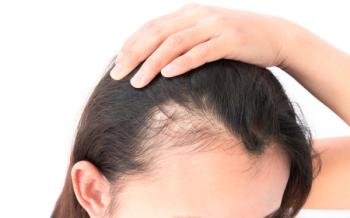
A 2017 study examines the long-term efficacy of JAK 1/3 inhibitor, tofacitinib, for treatment of alopecia areata and its variants.

This week, consumer magazines highlight the science behind the longevity movement for aging, dermarolling for hair loss, and more.

This week in aesthetics, we highlight collagen supplements for anti-aging, plastic surgery safety during COVID-19, and more.

Merz and Candela announce the launch of a commercial collaboration to provide one of the broadest medical aesthetics portfolios in the world.